| << Chapter < Page | Chapter >> Page > |
The temperature-programmed desorption (TPD) technique is often used to monitor surface interactions between adsorbed molecules and substrate surface. Utilizing the dependence on temperature is able to discriminate between processes with different activation parameters, such as activation energy, rate constant, reaction order and Arrhenius pre-exponential factorIn order to provide an example of the set-up and results from a TPD experiment we are going to use an ultra-high vacuum (UHV) chamber equipped with a quadrupole mass spectrometer to exemplify a typical surface gas-solid interaction and estimate several important kinetic parameters.
When we start to set up an apparatus for a typical surface TPD experiment, we should first think about how we can generate an extremely clean environment for the solid substrate and gas adsorbents. Ultra-high vacuum (UHV) is the most basic requirement for surface chemistry experiments. UHV is defined as a vacuum regime lower than 10 -9 Torr. At such a low pressure the mean free path of a gas molecule is approximately 40 Km, which means gas molecules will collide and react with sample substrate in the UHV chamber many times before colliding with each other, ensuring all interactions take place on the substrate surface.
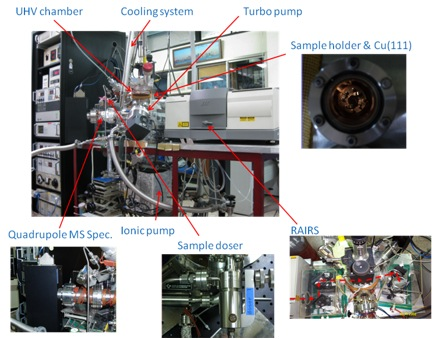
Most of time UHV chambers require the use of unusual materials in construction and by heating the entire system to ~180 °C for several hours baking to remove moisture and other trace adsorbed gases around the wall of the chamber in order to reach the ultra-high vacuum environment. Also, outgas from the substrate surface and other bulk materials should be minimized by careful selection of materials with low vapor pressures, such as stainless steel, for everything inside the UHV chamber. Thus bulk metal crystals are chosen as substrates to study interactions between gas adsorbates and crystal surface itself. [link] shows a schematic of a TPD system, while [link] shows a typical TPD instrument equipped with a quadrupole MS spectrometer and a reflection absorption infrared spectrometer (RAIRS).
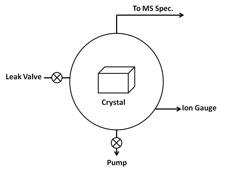
There is no single pump that can operate all the way from atmospheric pressure to UHV. Instead, a series of different pumps are used, according to the appropriate pressure range for each pump. Pumps are commonly used to achieve UHV include:
UHV pressures are measured with an ion-gauge, either a hot filament or an inverted magnetron type. Finally, special seals and gaskets must be used between components in a UHV system to prevent even trace leakage. Nearly all such seals are all metal, with knife edges on both sides cutting into a soft (e.g., copper) gasket. This all-metal seal can maintain system pressures down to ~10 -12 Torr.

Notification Switch
Would you like to follow the 'Physical methods in chemistry and nano science' conversation and receive update notifications?