| << Chapter < Page | Chapter >> Page > |
Permittivity (in the framework of electromagnetics) is a fundamental material property that describes how a material will affect, and be affected by, a time-varying electromagnetic field. The parameters of permittivity are often treated as a complex function of the applied electromagnetic field as complex numbers allow for the expression of magnitude and phase. The fundamental equation for the complex permittivity of a substance (ε s ) is given by [link] , where ε’ and ε’’ are the real and imaginary components, respectively, ω is the radial frequency (rad/s) and can be easily converted to frequency (Hertz, Hz) using [link] .
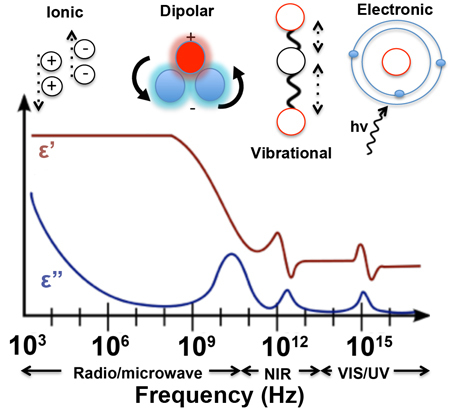
Specifically, the real and imaginary parameters defined within the complex permittivity equation describe how a material will store electromagnetic energy and dissipate that energy as heat. The processes that influence the response of a material to a time-varying electromagnetic field are frequency dependent and are generally classified as either ionic, dipolar, vibrational, or electronic in nature. These processes are highlighted as a function of frequency in [link] . Ionic processes refer to the general case of a charged ion moving back and forth in response a time-varying electric field, whilst dipolar processes correspond to the ‘flipping’ and ‘twisting’ of molecules, which have a permanent electric dipole moment such as that seen with a water molecule in a microwave oven. Examples of vibrational processes include molecular vibrations (e.g. symmetric and asymmetric) and associated vibrational-rotation states that are Infrared (IR) active. Electronic processes include optical and ultra-violet (UV) absorption and scattering phenomenon seen across the UV-visible range.
The most common relationship scientists that have with permittivity is through the concept of relative permittivity: the permittivity of a material relative to vacuum permittivity. Also known as the dielectric constant, the relative permittivity (ε r ) is given by [link] , where ε s is the permittivity of the substance and ε 0 is the permittivity of a vacuum (ε 0 = 8.85 x 10 -12 Farads/m). Although relative permittivity is in fact dynamic and a function of frequency, the dielectric constants are most often expressed for low frequency electric fields where the electric field is essential static in nature. [link] depicts the dielectric constants for a range of materials.
| Material | Relative Permittivity |
| Vacuum | 1 (by definition) |
| Air | 1.00058986 |
| Polytetrafluoroethylene (PTFE, Teflon) | 2.1 |
| Paper | 3.85 |
| Diamond | 5.5-10 |
| Methanol | 30 |
| Water | 80.1 |
| Titanium dioxide (TiO 2 ) | 86-173 |
| Strontium titanate (SrTiO 3 ) | 310 |
| Barium titanate (BaTiO 3 ) | 1,200-10,000 |
| Calcium copper titanate (CaCu 3 Ti 4 O 12 ) | >250,000 |

Notification Switch
Would you like to follow the 'Physical methods in chemistry and nano science' conversation and receive update notifications?