| << Chapter < Page | Chapter >> Page > |
Dynamic light scattering (DLS), which is also known as photon correlation spectroscopy (PCS) or quasi-elastic light scattering (QLS), is a spectroscopy method used in the fields of chemistry, biochemistry, and physics to determine the size distribution of particles (polymers, proteins, colloids, etc.) in solution or suspension. In the DLS experiment, normally a laser provides the monochromatic incident light, which impinges onto a solution with small particles in Brownian motion. And then through the Rayleigh scattering process, particles whose sizes are sufficiently small compared to the wavelength of the incident light will diffract the incident light in all direction with different wavelengths and intensities as a function of time. Since the scattering pattern of the light is highly correlated to the size distribution of the analyzed particles, the size-related information of the sample could be then acquired by mathematically processing the spectral characteristics of the scattered light.
Herein a brief introduction of basic theories of DLS will be demonstrated, followed by descriptions and guidance on the instrument itself and the sample preparation and measurement process. Finally, data analysis of the DLS measurement, and the applications of DLS as well as the comparison against other size-determine techniques will be shown and summarized.
The theory of DLS can be introduced utilizing a model system of spherical particles in solution. According to the Rayleigh scattering ( [link] ), when a sample of particles with diameter smaller than the wavelength of the incident light, each particle will diffract the incident light in all directions, while the intensity I is determined by [link] , where I 0 and λ is the intensity and wavelength of the unpolarized incident light, R is the distance to the particle, θ is the scattering angel, n is the refractive index of the particle, and r is the radius of the particle.
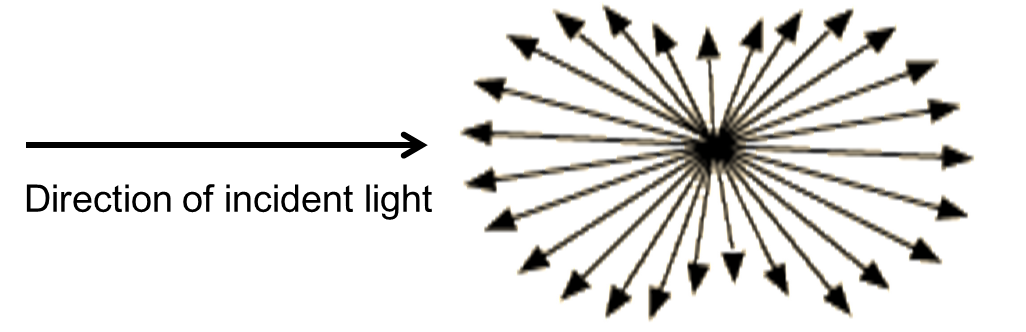
If that diffracted light is projected as an image onto a screen, it will generate a “speckle" pattern ( [link] ); the dark areas represent regions where the diffracted light from the particles arrives out of phase interfering destructively and the bright area represent regions where the diffracted light arrives in phase interfering constructively.

In practice, particle samples are normally not stationary but moving randomly due to collisions with solvent molecules as described by the Brownian motion, [link] , where is the mean squared displacement in time t , and D is the diffusion constant, which is related to the hydrodynamic radius a of the particle according to the Stokes-Einstein equation, [link] , where k B is Boltzmann constant, T is the temperature, and μ is viscosity of the solution. Importantly, for a system undergoing Brownian motion, small particles should diffuse faster than large ones.

Notification Switch
Would you like to follow the 'Physical methods in chemistry and nano science' conversation and receive update notifications?