| << Chapter < Page | Chapter >> Page > |
Fluoride is added into drinking water and toothpaste to prevent dental caries and thus the determination of its concentration is of great importance to human health. Here, we will give some data and calculations to show how the concentration of fluoride ion is determined and have a glance at how relevant ISE is to our daily life. According to Nernst equation, EQ ABOVE, in this case n = 1, T = 25 °C and E 0 , R, F are constants and thus this equation can be simplied as EQ.
The first step is to obtain a calibration curve for fluoride ion and this can be done by preparing several fluoride standard solution with known concentration ( [link] ) and making a plot of E versus log C ( [link] ).
| Concentration (mg/L) | log C | E (mV) |
| 200.0 | 2.301 | -35.6 |
| 100.0 | 2.000 | -17.8 |
| 50.00 | 1.699 | 0.4 |
| 25.00 | 1.398 | 16.8 |
| 12.50 | 1.097 | 34.9 |
| 6.250 | 0.796 | 52.8 |
| 3.125 | 0.495 | 70.4 |
| 1.563 | 0.194 | 89.3 |
| 0.781 | 0.107 | 107.1 |
| 0.391 | 0.408 | 125.5 |
| 0.195 | 0.709 | 142.9 |
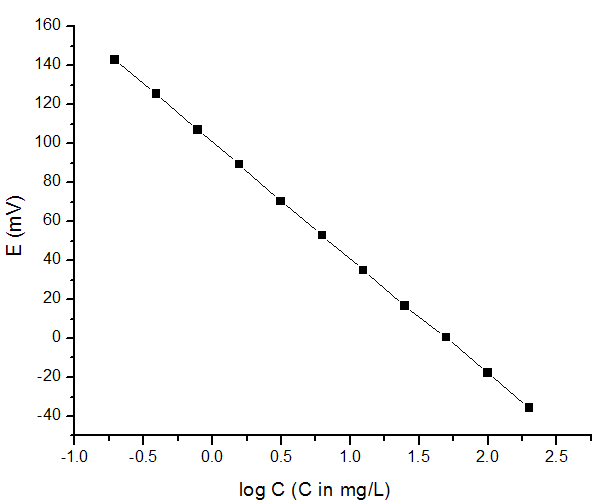
From the plot in [link] , we can clearly identify the linear relationship between E versus log C with slope measeured at -59.4 mV, which is very closed to the theoretical value -59.2 mV at 25 °C. This plot can give the concentration of any solution containing fluoride ion within the range of 0.195 mg/L and 200 mg/L by measuring the potential of the unknown solution.
Though ISE is a cost-effective and useful technique, it has some drawbacks that cannot be avoided. The selective ion membrane only allows the measured ions to pass and thus the potential is only determined by this particular ion. However, the truth is there is no such membrane that only permits the passage of one ion, and so there are cases when there are more than one ions that can pass the membrane. As a result, the measured potential are affected by the passage of the “unwanted” ions. Also, because of its dependence on ion selective membrane, one ISE is only suitable for one ion and this may be inconvenient sometimes. Another problem worth noticing is that ion selective measures the concentration of ions in equilibrium at the surface of the membrane surface. This does matter much if the solution is dilute but at higher concentrations, the inter-ionic interactions between the ions in the solution tend to decrease the mobility of ions and thus the concentration near the membrane would be lower than that in the bulk. This is one source of inaccuracy of ISE. To better analyze the results of ISE, we have to be aware of these inherent limitations of it.

Notification Switch
Would you like to follow the 'Physical methods in chemistry and nano science' conversation and receive update notifications?