| << Chapter < Page | Chapter >> Page > |
Check Your Understanding At the surface between air and water, light rays can go from air to water and from water to air. For which ray is there no possibility of total internal reflection?
air to water, because the condition that the second medium must have a smaller index of refraction is not satisfied
In the photo that opens this chapter, the image of a swimmer underwater is captured by a camera that is also underwater. The swimmer in the upper half of the photograph, apparently facing upward, is, in fact, a reflected image of the swimmer below. The circular ripple near the photograph’s center is actually on the water surface. The undisturbed water surrounding it makes a good reflecting surface when viewed from below, thanks to total internal reflection. However, at the very top edge of this photograph, rays from below strike the surface with incident angles less than the critical angle, allowing the camera to capture a view of activities on the pool deck above water.
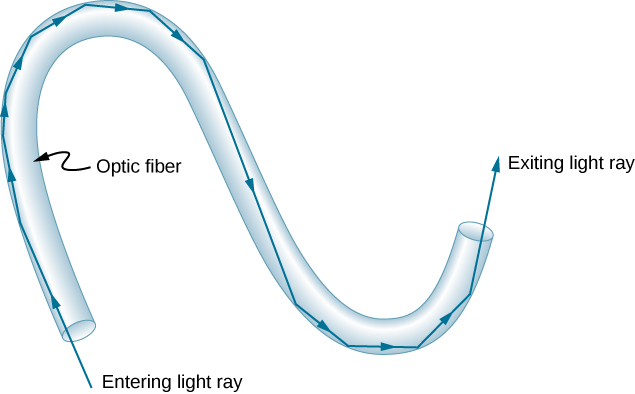
Fiber optics is one application of total internal reflection that is in wide use. In communications, it is used to transmit telephone, internet, and cable TV signals. Fiber optics employs the transmission of light down fibers of plastic or glass. Because the fibers are thin, light entering one is likely to strike the inside surface at an angle greater than the critical angle and, thus, be totally reflected ( [link] ). The index of refraction outside the fiber must be smaller than inside. In fact, most fibers have a varying refractive index to allow more light to be guided along the fiber through total internal refraction. Rays are reflected around corners as shown, making the fibers into tiny light pipes.
Bundles of fibers can be used to transmit an image without a lens, as illustrated in [link] . The output of a device called an endoscope is shown in [link] (b). Endoscopes are used to explore the interior of the body through its natural orifices or minor incisions. Light is transmitted down one fiber bundle to illuminate internal parts, and the reflected light is transmitted back out through another bundle to be observed.
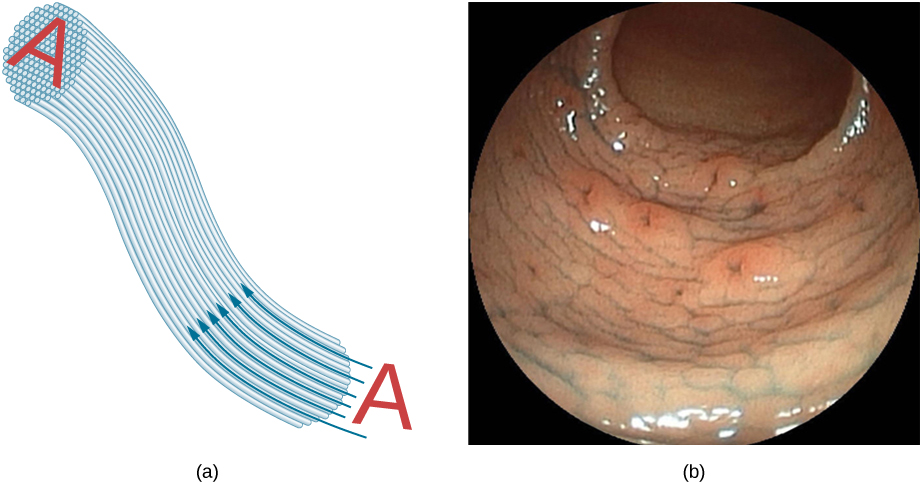
Fiber optics has revolutionized surgical techniques and observations within the body, with a host of medical diagnostic and therapeutic uses. Surgery can be performed, such as arthroscopic surgery on a knee or shoulder joint, employing cutting tools attached to and observed with the endoscope. Samples can also be obtained, such as by lassoing an intestinal polyp for external examination. The flexibility of the fiber optic bundle allows doctors to navigate it around small and difficult-to-reach regions in the body, such as the intestines, the heart, blood vessels, and joints. Transmission of an intense laser beam to burn away obstructing plaques in major arteries, as well as delivering light to activate chemotherapy drugs, are becoming commonplace. Optical fibers have in fact enabled microsurgery and remote surgery where the incisions are small and the surgeon’s fingers do not need to touch the diseased tissue.

Notification Switch
Would you like to follow the 'University physics volume 3' conversation and receive update notifications?