| << Chapter < Page | Chapter >> Page > |
Check Your Understanding What happens to the ground state energy of an electron if the dimensions of the solid increase?
It decreases.
Often, we are not interested in the total number of particles in all states, but rather the number of particles dN with energies in a narrow energy interval. This value can be expressed by
where n ( E ) is the electron number density , or the number of electrons per unit volume; g ( E ) is the density of states , or the number of allowed quantum states per unit energy; dE is the size of the energy interval; and F is the Fermi factor . The Fermi factor is the probability that the state will be filled. For example, if g ( E ) dE is 100 available states, but F is only , then the number of particles in this narrow energy interval is only five. Finding g ( E ) requires solving Schrödinger’s equation (in three dimensions) for the allowed energy levels. The calculation is involved even for a crude model, but the result is simple:
where V is the volume of the solid, is the mass of the electron, and E is the energy of the state. Notice that the density of states increases with the square root of the energy. More states are available at high energy than at low energy. This expression does not provide information of the density of the electrons in physical space, but rather the density of energy levels in “energy space.” For example, in our study of the atomic structure, we learned that the energy levels of a hydrogen atom are much more widely spaced for small energy values (near than ground state) than for larger values.
This equation tells us how many electron states are available in a three-dimensional metallic solid. However, it does not tell us how likely these states will be filled. Thus, we need to determine the Fermi factor, F . Consider the simple case of . From classical physics, we expect that all the electrons would simply go into the ground state to achieve the lowest possible energy. However, this violates Pauli’s exclusion principle, which states that no two electrons can be in the same quantum state. Hence, when we begin filling the states with electrons, the states with lowest energy become occupied first, then states with progressively higher energies. The last electron we put in has the highest energy. This energy is the Fermi energy of the free electron gas. A state with energy is occupied by a single electron, and a state with energy is unoccupied. To describe this in terms of a probability F ( E ) that a state of energy E is occupied, we write for :
The density of states, Fermi factor, and electron number density are plotted against energy in [link] .
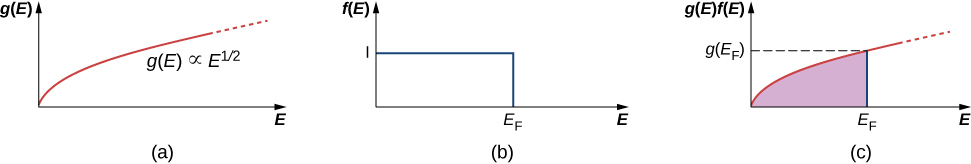
A few notes are in order. First, the electron number density (last row) distribution drops off sharply at the Fermi energy. According to the theory, this energy is given by

Notification Switch
Would you like to follow the 'University physics volume 3' conversation and receive update notifications?