| << Chapter < Page | Chapter >> Page > |
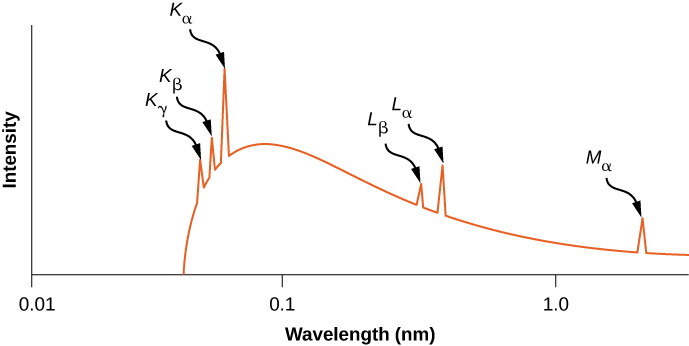
Based on the relationship , the frequency of the X-ray is
Hence, the transition L K in aluminum produces X-ray radiation.
X-ray production provides an important test of quantum mechanics. According to the Bohr model, the energy of a X-ray depends on the nuclear charge or atomic number, Z . If Z is large, Coulomb forces in the atom are large, energy differences ( ) are large, and, therefore, the energy of radiated photons is large. To illustrate, consider a single electron in a multi-electron atom. Neglecting interactions between the electrons, the allowed energy levels are
where n = 1, 2, …and Z is the atomic number of the nucleus. However, an electron in the L ( ) shell “sees” a charge , because one electron in the K shell shields the nuclear charge. (Recall that there is only one electron in the K shell because the other electron was “knocked out.”) Therefore, the approximate energies of the electron in the L and K shells are
The energy carried away by a photon in a transition from the L shell to the K shell is therefore
where Z is the atomic number. In general, the X-ray photon energy for a transition from an outer shell to the K shell is
or
where f is the frequency of a X-ray. This equation is Moseley’s law . For large values of Z , we have approximately
This prediction can be checked by measuring f for a variety of metal targets. This model is supported if a plot of Z versus data (called a Moseley plot ) is linear. Comparison of model predictions and experimental results, for both the K and L series, is shown in [link] . The data support the model that X-rays are produced when an outer shell electron drops in energy to fill a vacancy in an inner shell.
Check Your Understanding X-rays are produced by bombarding a metal target with high-energy electrons. If the target is replaced by another with two times the atomic number, what happens to the frequency of X-rays?
frequency quadruples

Notification Switch
Would you like to follow the 'University physics volume 3' conversation and receive update notifications?