| << Chapter < Page | Chapter >> Page > |
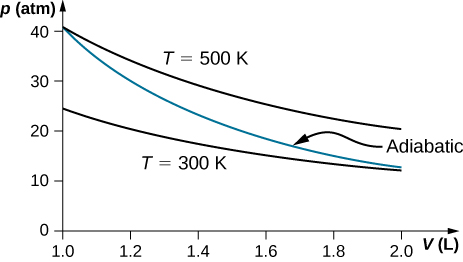
On an adiabatic process of an ideal gas pressure, volume and temperature change such that is constant with for monatomic gas such as helium and for diatomic gas such as hydrogen at room temperature. Use numerical values to plot two isotherms of 1 mol of helium gas using ideal gas law and two adiabatic processes mediating between them. Use for your plot.
Two moles of a monatomic ideal gas such as helium is compressed adiabatically and reversibly from a state (3 atm, 5 L) to a state with pressure 4 atm. (a) Find the volume and temperature of the final state. (b) Find the temperature of the initial state of the gas. (c) Find the work done by the gas in the process. (d) Find the change in internal energy of the gas in the process.
Consider the process shown below. During steps AB and BC , 3600 J and 2400 J of heat, respectively, are added to the system. (a) Find the work done in each of the processes AB , BC , AD , and DC . (b) Find the internal energy change in processes AB and BC . (c) Find the internal energy difference between states C and A . (d) Find the total heat added in the ADC process. (e) From the information given, can you find the heat added in process AD ? Why or why not?
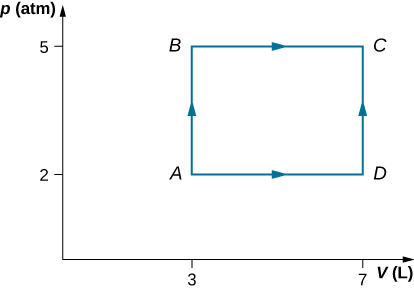
a. b. c. d. e. No, because heat was added for both parts AD and DC . There is not enough information to figure out how much is from each segment of the path.
A car tire contains of air at a pressure of (about 32 psi). How much more internal energy does this gas have than the same volume has at zero gauge pressure (which is equivalent to normal atmospheric pressure)?
A helium-filled toy balloon has a gauge pressure of 0.200 atm and a volume of 10.0 L. How much greater is the internal energy of the helium in the balloon than it would be at zero gauge pressure?
300 J
Steam to drive an old-fashioned steam locomotive is supplied at a constant gauge pressure of (about 250 psi) to a piston with a 0.200-m radius. (a) By calculating , find the work done by the steam when the piston moves 0.800 m. Note that this is the net work output, since gauge pressure is used. (b) Now find the amount of work by calculating the force exerted times the distance traveled. Is the answer the same as in part (a)?
A hand-driven tire pump has a piston with a 2.50-cm diameter and a maximum stroke of 30.0 cm. (a) How much work do you do in one stroke if the average gauge pressure is (about 35 psi)? (b) What average force do you exert on the piston, neglecting friction and gravitational force?
a. 59.5 J; b. 170 N
Calculate the net work output of a heat engine following path ABCDA as shown below.
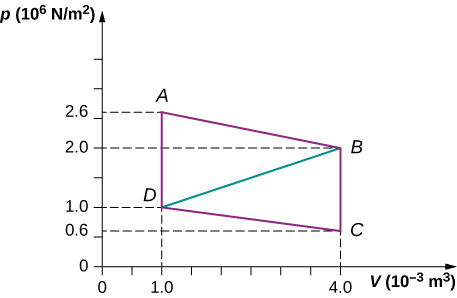
What is the net work output of a heat engine that follows path ABDA in the preceding problem with a straight line from B to D ? Why is the work output less than for path ABCDA ?
Five moles of a monatomic ideal gas in a cylinder at is expanded isothermally from a volume of 5 L to 10 L. (a) What is the change in internal energy? (b) How much work was done on the gas in the process? (c) How much heat was transferred to the gas?

Notification Switch
Would you like to follow the 'University physics volume 2' conversation and receive update notifications?