| << Chapter < Page | Chapter >> Page > |
A 300-W heat pump operates between the ground, whose temperature is , and the interior of a house at . What is the maximum amount of heat per hour that the heat pump can supply to the house?
An engineer must design a refrigerator that does 300 J of work per cycle to extract 2100 J of heat per cycle from a freezer whose temperature is . What is the maximum air temperature for which this condition can be met? Is this a reasonable condition to impose on the design?
A Carnot engine employs 1.5 mol of nitrogen gas as a working substance, which is considered as an ideal diatomic gas with at the working temperatures of the engine. The Carnot cycle goes in the cycle ABCDA with AB being an isothermal expansion. The volume at points A and C of the cycle are and 0.15 L, respectively. The engine operates between two thermal baths of temperature 500 K and 300 K. (a) Find the values of volume at B and D . (b) How much heat is absorbed by the gas in the AB isothermal expansion? (c) How much work is done by the gas in the AB isothermal expansion? (d) How much heat is given up by the gas in the CD isothermal expansion? (e) How much work is done by the gas in the CD isothermal compression? (f) How much work is done by the gas in the BC adiabatic expansion? (g) How much work is done by the gas in the DA adiabatic compression? (h) Find the value of efficiency of the engine based on the net work and heat input. Compare this value to the efficiency of a Carnot engine based on the temperatures of the two baths.
a. b. 13,000 J; c. 13,000 J; d. –8,000 J; e. –8,000 J; f. 6200 J; g. –6200 J; h. ; with temperatures efficiency is , which is off likely by rounding errors.
A 5.0-kg wood block starts with an initial speed of 8.0 m/s and slides across the floor until friction stops it. Estimate the resulting change in entropy of the universe. Assume that everything stays at a room temperature of .
A system consisting of 20.0 mol of a monoatomic ideal gas is cooled at constant pressure from a volume of 50.0 L to 10.0 L. The initial temperature was 300 K. What is the change in entropy of the gas?
–670 J/K
A glass beaker of mass 400 g contains 500 g of water at . The beaker is heated reversibly so that the temperature of the beaker and water rise gradually to . Find the change in entropy of the beaker and water together.
A Carnot engine operates between and baths and produces 300 kJ of energy in each cycle. Find the change in entropy of the (a) hot bath and (b) cold bath, in each Carnot cycle?
a. –570 J/K; b. 570 J/K
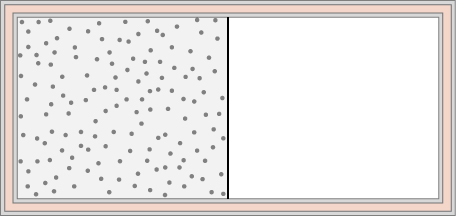
An ideal gas at temperature T is stored in the left half of an insulating container of volume V using a partition of negligible volume (see below). What is the entropy change per mole of the gas in each of the following cases? (a) The partition is suddenly removed and the gas quickly fills the entire container. (b) A tiny hole is punctured in the partition and after a long period, the gas reaches an equilibrium state such that there is no net flow through the hole. (c) The partition is moved very slowly and adiabatically all the way to the right wall so that the gas finally fills the entire container.

Notification Switch
Would you like to follow the 'University physics volume 2' conversation and receive update notifications?