| << Chapter < Page | Chapter >> Page > |
In an ideal gas (see The Kinetic Theory of Gases ), the equation for the speed of sound is
where is the adiabatic index, is the gas constant, is the absolute temperature in kelvins, and M is the molecular mass. In general, the more rigid (or less compressible) the medium, the faster the speed of sound. This observation is analogous to the fact that the frequency of simple harmonic motion is directly proportional to the stiffness of the oscillating object as measured by k , the spring constant. The greater the density of a medium, the slower the speed of sound. This observation is analogous to the fact that the frequency of a simple harmonic motion is inversely proportional to m , the mass of the oscillating object. The speed of sound in air is low, because air is easily compressible. Because liquids and solids are relatively rigid and very difficult to compress, the speed of sound in such media is generally greater than in gases.
| Medium | v (m/s) |
|---|---|
| Gases at | |
| Air | 331 |
| Carbon dioxide | 259 |
| Oxygen | 316 |
| Helium | 965 |
| Hydrogen | 1290 |
| Liquids at | |
| Ethanol | 1160 |
| Mercury | 1450 |
| Water, fresh | 1480 |
| Sea Water | 1540 |
| Human tissue | 1540 |
| Solids (longitudinal or bulk) | |
| Vulcanized rubber | 54 |
| Polyethylene | 920 |
| Marble | 3810 |
| Glass, Pyrex | 5640 |
| Lead | 1960 |
| Aluminum | 5120 |
| Steel | 5960 |
Because the speed of sound depends on the density of the material, and the density depends on the temperature, there is a relationship between the temperature in a given medium and the speed of sound in the medium. For air at sea level, the speed of sound is given by
where the temperature in the first equation (denoted as ) is in degrees Celsius and the temperature in the second equation (denoted as ) is in kelvins. The speed of sound in gases is related to the average speed of particles in the gas, where is the Boltzmann constant and m is the mass of each (identical) particle in the gas. Note that v refers to the speed of the coherent propagation of a disturbance (the wave), whereas describes the speeds of particles in random directions. Thus, it is reasonable that the speed of sound in air and other gases should depend on the square root of temperature. While not negligible, this is not a strong dependence. At , the speed of sound is 331 m/s, whereas at , it is 343 m/s, less than a increase. [link] shows how a bat uses the speed of sound to sense distances.
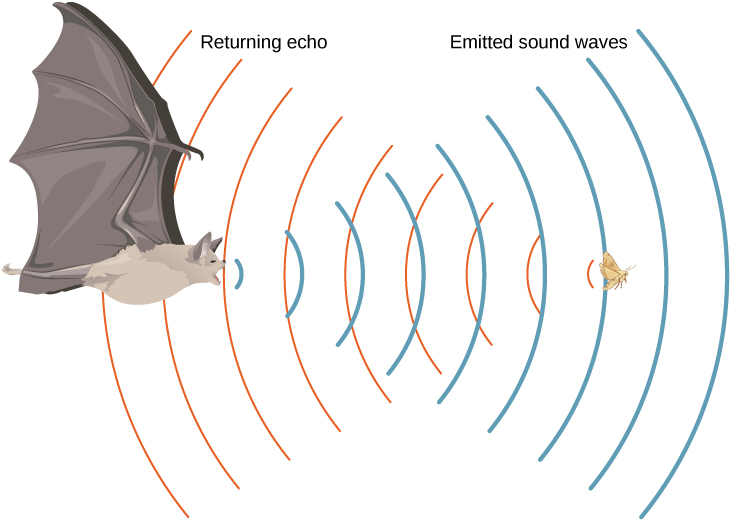
As stated earlier, the speed of sound in a medium depends on the medium and the state of the medium. The derivation of the equation for the speed of sound in air starts with the mass flow rate and continuity equation discussed in Fluid Mechanics .
Consider fluid flow through a pipe with cross-sectional area A ( [link] ). The mass in a small volume of length x of the pipe is equal to the density times the volume, or The mass flow rate is

Notification Switch
Would you like to follow the 'University physics volume 1' conversation and receive update notifications?