| << Chapter < Page | Chapter >> Page > |
In this chapter, we have studied energy. We learned that energy can take different forms and can be transferred from one form to another. You will find that energy is discussed in many everyday, as well as scientific, contexts, because it is involved in all physical processes. It will also become apparent that many situations are best understood, or most easily conceptualized, by considering energy. So far, no experimental results have contradicted the conservation of energy. In fact, whenever measurements have appeared to conflict with energy conservation, new forms of energy have been discovered or recognized in accordance with this principle.
What are some other forms of energy? Many of these are covered in later chapters (also see [link] ), but let’s detail a few here:
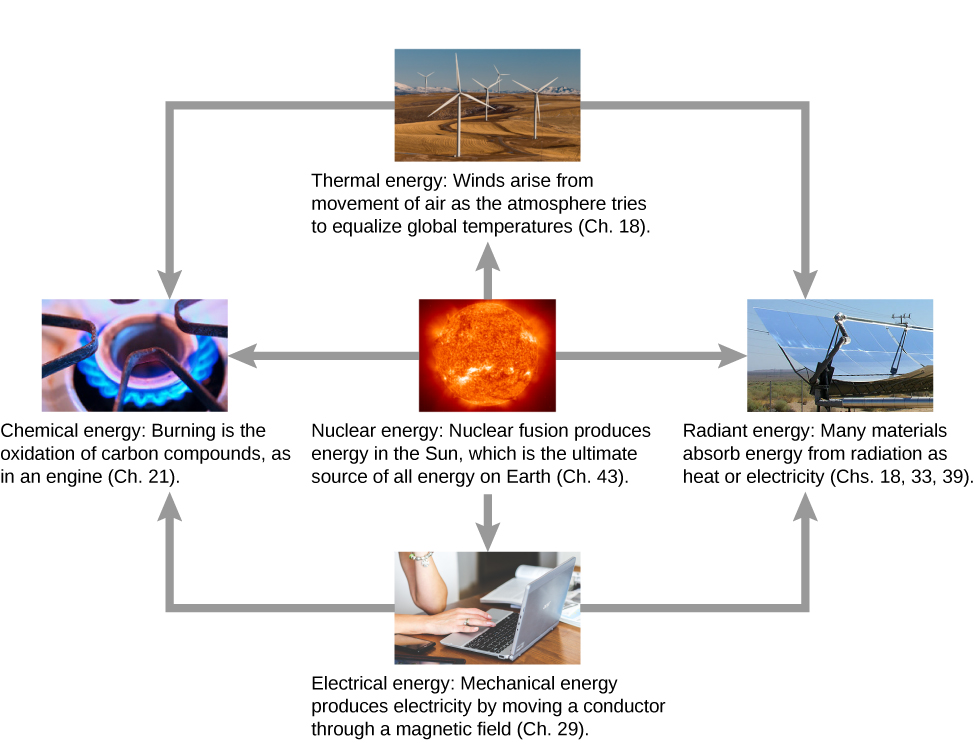
The transformation of energy from one form into another happens all the time. The chemical energy in food is converted into thermal energy through metabolism; light energy is converted into chemical energy through photosynthesis. Another example of energy conversion occurs in a solar cell. Sunlight impinging on a solar cell produces electricity, which can be used to run electric motors or heat water. In an example encompassing many steps, the chemical energy contained in coal is converted into thermal energy as it burns in a furnace, to transform water into steam, in a boiler. Some of the thermal energy in the steam is then converted into mechanical energy as it expands and spins a turbine, which is connected to a generator to produce electrical energy. In these examples, not all of the initial energy is converted into the forms mentioned, because some energy is always transferred to the environment.

Notification Switch
Would you like to follow the 'University physics volume 1' conversation and receive update notifications?