| << Chapter < Page | Chapter >> Page > |
In contrast, atoms in gases are separated by large distances, and the forces between atoms in a gas are therefore very weak, except when the atoms collide with one another. This makes gases relatively easy to compress and allows them to flow (which makes them fluids). When placed in an open container, gases, unlike liquids, will escape.
In this chapter, we generally refer to both gases and liquids simply as fluids, making a distinction between them only when they behave differently. There exists one other phase of matter, plasma , which exists at very high temperatures. At high temperatures, molecules may disassociate into atoms, and atoms disassociate into electrons (with negative charges) and protons (with positive charges), forming a plasma. Plasma will not be discussed in depth in this chapter because plasma has very different properties from the three other common phases of matter, discussed in this chapter, due to the strong electrical forces between the charges.
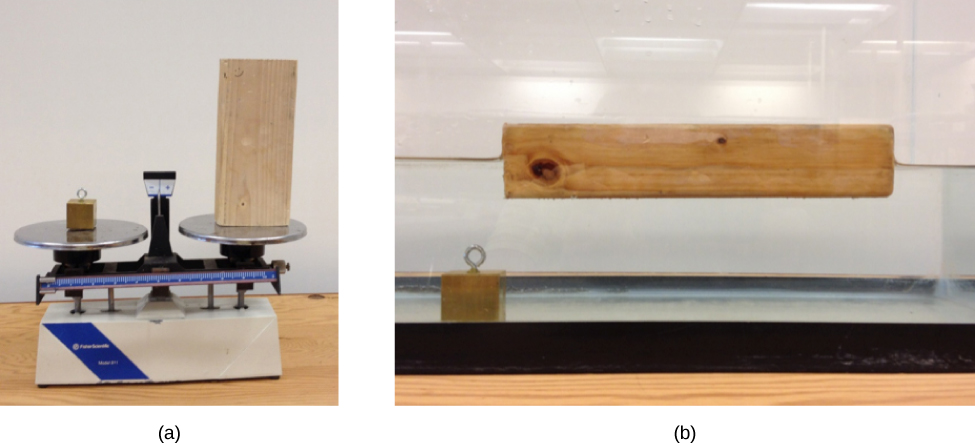
Suppose a block of brass and a block of wood have exactly the same mass. If both blocks are dropped in a tank of water, why does the wood float and the brass sink ( [link] )? This occurs because the brass has a greater density than water, whereas the wood has a lower density than water.
Density is an important characteristic of substances. It is crucial, for example, in determining whether an object sinks or floats in a fluid.
The average density of a substance or object is defined as its mass per unit volume,
where the Greek letter (rho) is the symbol for density, m is the mass, and V is the volume.
The SI unit of density is . [link] lists some representative values. The cgs unit of density is the gram per cubic centimeter, , where
The metric system was originally devised so that water would have a density of , equivalent to . Thus, the basic mass unit, the kilogram, was first devised to be the mass of 1000 mL of water, which has a volume of .
| Solids
( ) |
Liquids
( ) |
Gases
( 101.3 kPa) | |||
|---|---|---|---|---|---|
| Substance | Substance | Substance | |||
| Aluminum | Benzene | Air | |||
| Bone | Blood | Carbon dioxide | |||
| Brass | Ethyl alcohol | Carbon monoxide | |||
| Concrete | Gasoline | Helium | |||
| Copper | Glycerin | Hydrogen | |||
| Cork | Mercury | Methane | |||
| Earth’s crust | Olive oil | Nitrogen | |||
| Glass | Nitrous oxide | ||||
| Gold | Oxygen | ||||
| Granite | |||||
| Iron | |||||
| Lead | |||||
| Oak | |||||
| Pine | |||||
| Platinum | |||||
| Polystyrene | |||||
| Tungsten | |||||
| Uranium |
As you can see by examining [link] , the density of an object may help identify its composition. The density of gold, for example, is about 2.5 times the density of iron, which is about 2.5 times the density of aluminum. Density also reveals something about the phase of the matter and its substructure. Notice that the densities of liquids and solids are roughly comparable, consistent with the fact that their atoms are in close contact. The densities of gases are much less than those of liquids and solids, because the atoms in gases are separated by large amounts of empty space. The gases are displayed for a standard temperature of and a standard pressure of 101.3 kPa, and there is a strong dependence of the densities on temperature and pressure. The densities of the solids and liquids displayed are given for the standard temperature of and the densities of solids and liquids depend on the temperature. The density of solids and liquids normally increase with decreasing temperature.

Notification Switch
Would you like to follow the 'University physics volume 1' conversation and receive update notifications?