| << Chapter < Page | Chapter >> Page > |
All three curves on the phase diagram meet at a single point, the triple point , where all three phases exist in equilibrium. For water, the triple point occurs at 273.16 K , and is a more accurate calibration temperature than the melting point of water at 1.00 atm, or 273.15 K . See [link] for the triple point values of other substances.
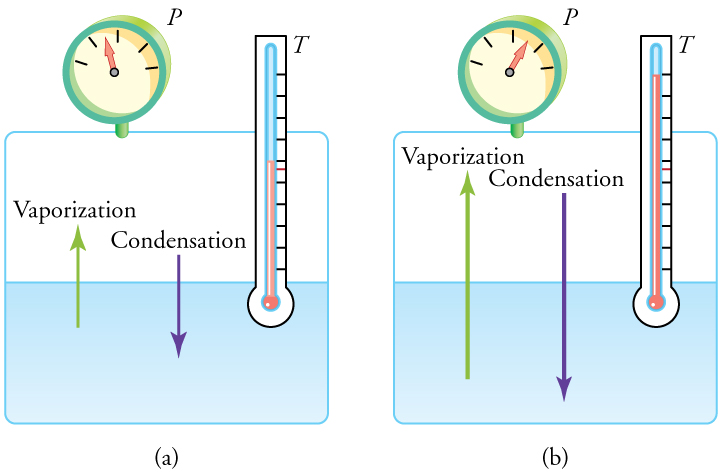
Liquid and gas phases are in equilibrium at the boiling temperature. (See [link] .) If a substance is in a closed container at the boiling point, then the liquid is boiling and the gas is condensing at the same rate without net change in their relative amount. Molecules in the liquid escape as a gas at the same rate at which gas molecules stick to the liquid, or form droplets and become part of the liquid phase. The combination of temperature and pressure has to be “just right”; if the temperature and pressure are increased, equilibrium is maintained by the same increase of boiling and condensation rates.
| Substance | Temperature | Pressure | ||
|---|---|---|---|---|
| Water | 273.16 | 0.01 | 0.00600 | |
| Carbon dioxide | 216.55 | −56.60 | 5.11 | |
| Sulfur dioxide | 197.68 | −75.47 | 0.0167 | |
| Ammonia | 195.40 | −77.75 | 0.0600 | |
| Nitrogen | 63.18 | −210.0 | 0.124 | |
| Oxygen | 54.36 | −218.8 | 0.00151 | |
| Hydrogen | 13.84 | −259.3 | 0.0697 |
One example of equilibrium between liquid and gas is that of water and steam at and 1.00 atm. This temperature is the boiling point at that pressure, so they should exist in equilibrium. Why does an open pot of water at boil completely away? The gas surrounding an open pot is not pure water: it is mixed with air. If pure water and steam are in a closed container at and 1.00 atm, they would coexist—but with air over the pot, there are fewer water molecules to condense, and water boils. What about water at and 1.00 atm? This temperature and pressure correspond to the liquid region, yet an open glass of water at this temperature will completely evaporate. Again, the gas around it is air and not pure water vapor, so that the reduced evaporation rate is greater than the condensation rate of water from dry air. If the glass is sealed, then the liquid phase remains. We call the gas phase a vapor when it exists, as it does for water at , at a temperature below the boiling temperature.
Explain why a cup of water (or soda) with ice cubes stays at , even on a hot summer day.
The ice and liquid water are in thermal equilibrium, so that the temperature stays at the freezing temperature as long as ice remains in the liquid. (Once all of the ice melts, the water temperature will start to rise.)

Notification Switch
Would you like to follow the 'College physics' conversation and receive update notifications?