| << Chapter < Page | Chapter >> Page > |
Therapeutic applications of ionizing radiation, called radiation therapy or radiotherapy , have existed since the discovery of x-rays and nuclear radioactivity. Today, radiotherapy is used almost exclusively for cancer therapy, where it saves thousands of lives and improves the quality of life and longevity of many it cannot save. Radiotherapy may be used alone or in combination with surgery and chemotherapy (drug treatment) depending on the type of cancer and the response of the patient. A careful examination of all available data has established that radiotherapy’s beneficial effects far outweigh its long-term risks.
The earliest uses of ionizing radiation on humans were mostly harmful, with many at the level of snake oil as seen in [link] . Radium-doped cosmetics that glowed in the dark were used around the time of World War I. As recently as the 1950s, radon mine tours were promoted as healthful and rejuvenating—those who toured were exposed but gained no benefits. Radium salts were sold as health elixirs for many years. The gruesome death of a wealthy industrialist, who became psychologically addicted to the brew, alerted the unsuspecting to the dangers of radium salt elixirs. Most abuses finally ended after the legislation in the 1950s.

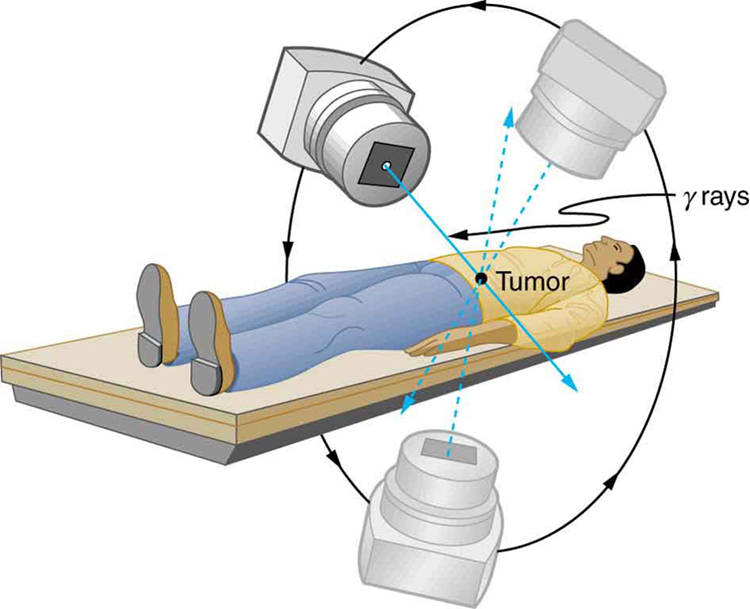
Radiotherapy is effective against cancer because cancer cells reproduce rapidly and, consequently, are more sensitive to radiation. The central problem in radiotherapy is to make the dose for cancer cells as high as possible while limiting the dose for normal cells. The ratio of abnormal cells killed to normal cells killed is called the therapeutic ratio , and all radiotherapy techniques are designed to enhance this ratio. Radiation can be concentrated in cancerous tissue by a number of techniques. One of the most prevalent techniques for well-defined tumors is a geometric technique shown in [link] . A narrow beam of radiation is passed through the patient from a variety of directions with a common crossing point in the tumor. This concentrates the dose in the tumor while spreading it out over a large volume of normal tissue. The external radiation can be x-rays, rays, or ionizing-particle beams produced by accelerators. Accelerator-produced beams of neutrons, , and heavy ions such as nitrogen nuclei have been employed, and these can be quite effective. These particles have larger QFs or RBEs and sometimes can be better localized, producing a greater therapeutic ratio. But accelerator radiotherapy is much more expensive and less frequently employed than other forms.

Notification Switch
Would you like to follow the 'College physics' conversation and receive update notifications?