| << Chapter < Page | Chapter >> Page > |
(1) Try floating a sewing needle on water. In order for this activity to work, the needle needs to be very clean as even the oil from your fingers can be sufficient to affect the surface properties of the needle. (2) Place the bristles of a paint brush into water. Pull the brush out and notice that for a short while, the bristles will stick together. The surface tension of the water surrounding the bristles is sufficient to hold the bristles together. As the bristles dry out, the surface tension effect dissipates. (3) Place a loop of thread on the surface of still water in such a way that all of the thread is in contact with the water. Note the shape of the loop. Now place a drop of detergent into the middle of the loop. What happens to the shape of the loop? Why? (4) Sprinkle pepper onto the surface of water. Add a drop of detergent. What happens? Why? (5) Float two matches parallel to each other and add a drop of detergent between them. What happens? Note: For each new experiment, the water needs to be replaced and the bowl washed to free it of any residual detergent.
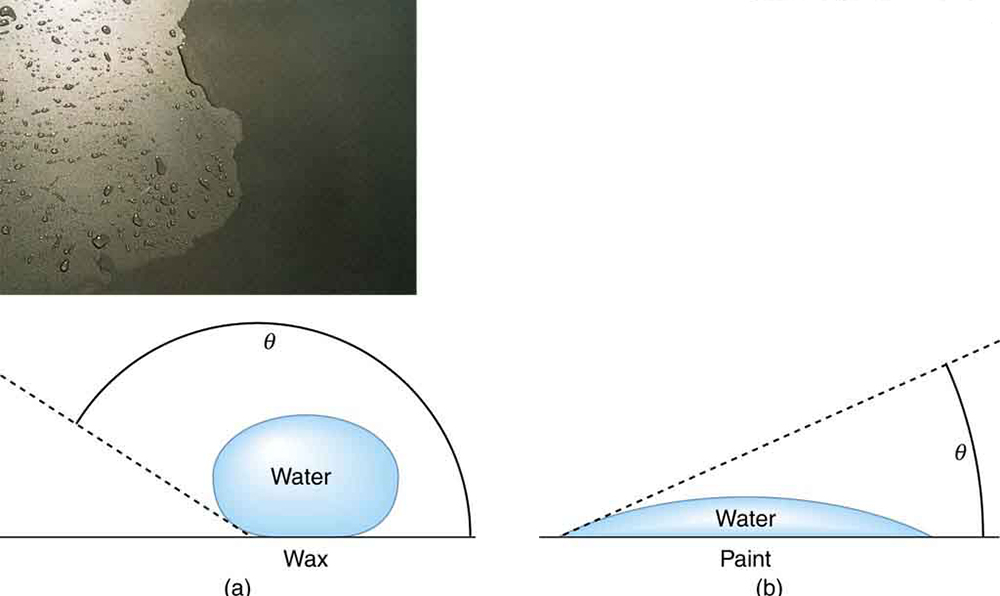
Why is it that water beads up on a waxed car but does not on bare paint? The answer is that the adhesive forces between water and wax are much smaller than those between water and paint. Competition between the forces of adhesion and cohesion are important in the macroscopic behavior of liquids. An important factor in studying the roles of these two forces is the angle between the tangent to the liquid surface and the surface. (See [link] .) The contact angle is directly related to the relative strength of the cohesive and adhesive forces. The larger the strength of the cohesive force relative to the adhesive force, the larger is, and the more the liquid tends to form a droplet. The smaller is, the smaller the relative strength, so that the adhesive force is able to flatten the drop. [link] lists contact angles for several combinations of liquids and solids.
The angle between the tangent to the liquid surface and the surface is called the contact angle.
One important phenomenon related to the relative strength of cohesive and adhesive forces is capillary action —the tendency of a fluid to be raised or suppressed in a narrow tube, or capillary tube . This action causes blood to be drawn into a small-diameter tube when the tube touches a drop.

Notification Switch
Would you like to follow the 'College physics for ap® courses' conversation and receive update notifications?