| << Chapter < Page | Chapter >> Page > |
[link] summarizes the comparative sizes of oxygen and sulfur.
| Element | Atomic radius (Å) | Covalent radius (Å) | Ionic radius (Å) | van der Waal radius (Å) |
| Oxygen | 0.48 | 0.66 | 1.40 | 1.52 |
| Sulfur | 0.88 | 1.05 | 1.84 | 1.80 |
Sulfur is less electronegative than oxygen (2.4 and 3.5, respectively) and as a consequence bonds to sulfur are less polar than the corresponding bonds to oxygen. One significant result in that with a less polar S-H bond the subsequent hydrogen bonding is weaker than observed with O-H analogs. A further consequence of the lower electronegativity is that the S-O bond is polar.
Sulfur forms a range of bonding types. As with oxygen the -2 oxidation state prevalent. For example, sulfur forms analogs of ethers, i.e., thioethers R-S-R. However, unlike oxygen, sulfur can form more than two covalent (non-dative) bonds, i.e., in compounds such as SF 4 and SF 6 .
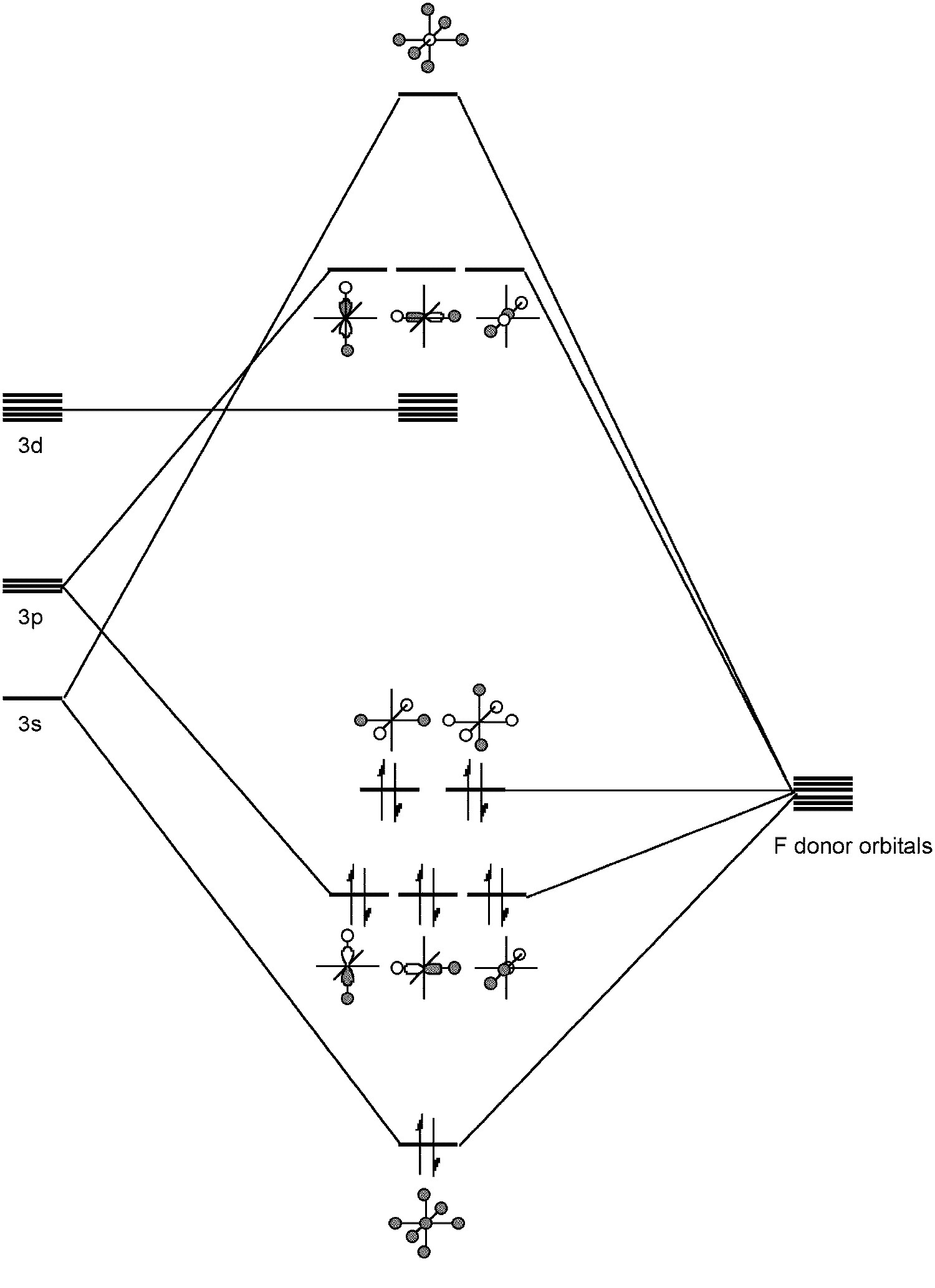
Such hypervalent compounds were originally thought be due to the inclusion of low energy d orbitals in hybrids (e.g., sp 3 d 2 for SF 6 ); however, a better picture involves a combination of s and p ortbitals in bonding ( [link] ). Any involvement of the d orbitals is limited to the polarization of the p orbitals rather than direct hydridization. In this regard SF 6 represents the archetypal hypervalent molecule. Finally, sulfur can form multiple bonds, e.g., Me 2 S=O.
Catenation is defined as the ability of a chemical element to form a long chain-like structure via a series of covalent bonds. Oxygen’s extent of catenation is limited to ozone (O 3 ) and peroxides (e.g., R-O-O-R). In contrast, the chemistry of sulfur is rich in the formation of multiple S-S bonds.
While elemental sulfur exists as a diatomic molecule (i.e., S 2 ) in the gas phase at high temperatures, sulfur vapor consists of a mixture of oligomers (S 3 to S 8 ) as a temperature dependant equilibrium. In the solid state the formation of Sn dominates, and sulfur exists as a range of polymorphs in which extended S-S bonding occurs in either rings of 6 to 20 atoms (e.g., [link] ) or chains (catenasulfur).
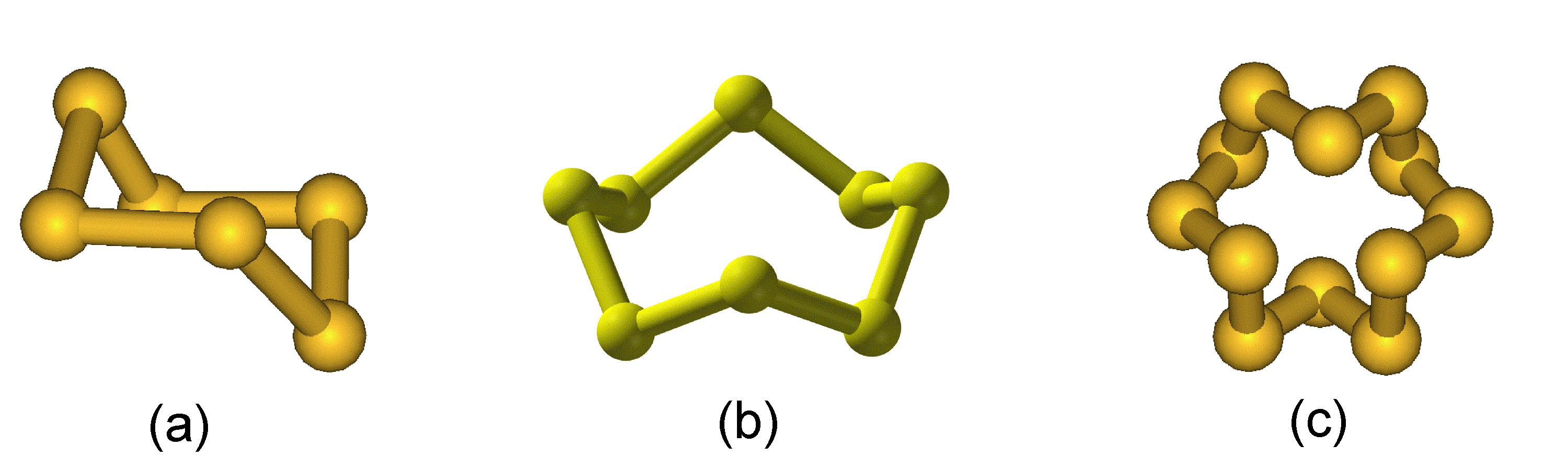
The higher level of catenation for sulfur is due to the greater strength of a S-S bond (226 kJ/mol) as compared to the O-O bond (142 kJ/mol). In general the homoleptic bond strength is expected to decrease going down a period of the Periodic Table. The reason for the unexpected weakness of the O-O bond is that the electronegative oxygen atoms repel each other and thus weaken the bond.

Notification Switch
Would you like to follow the 'Chemistry of the main group elements' conversation and receive update notifications?