| << Chapter < Page | Chapter >> Page > |
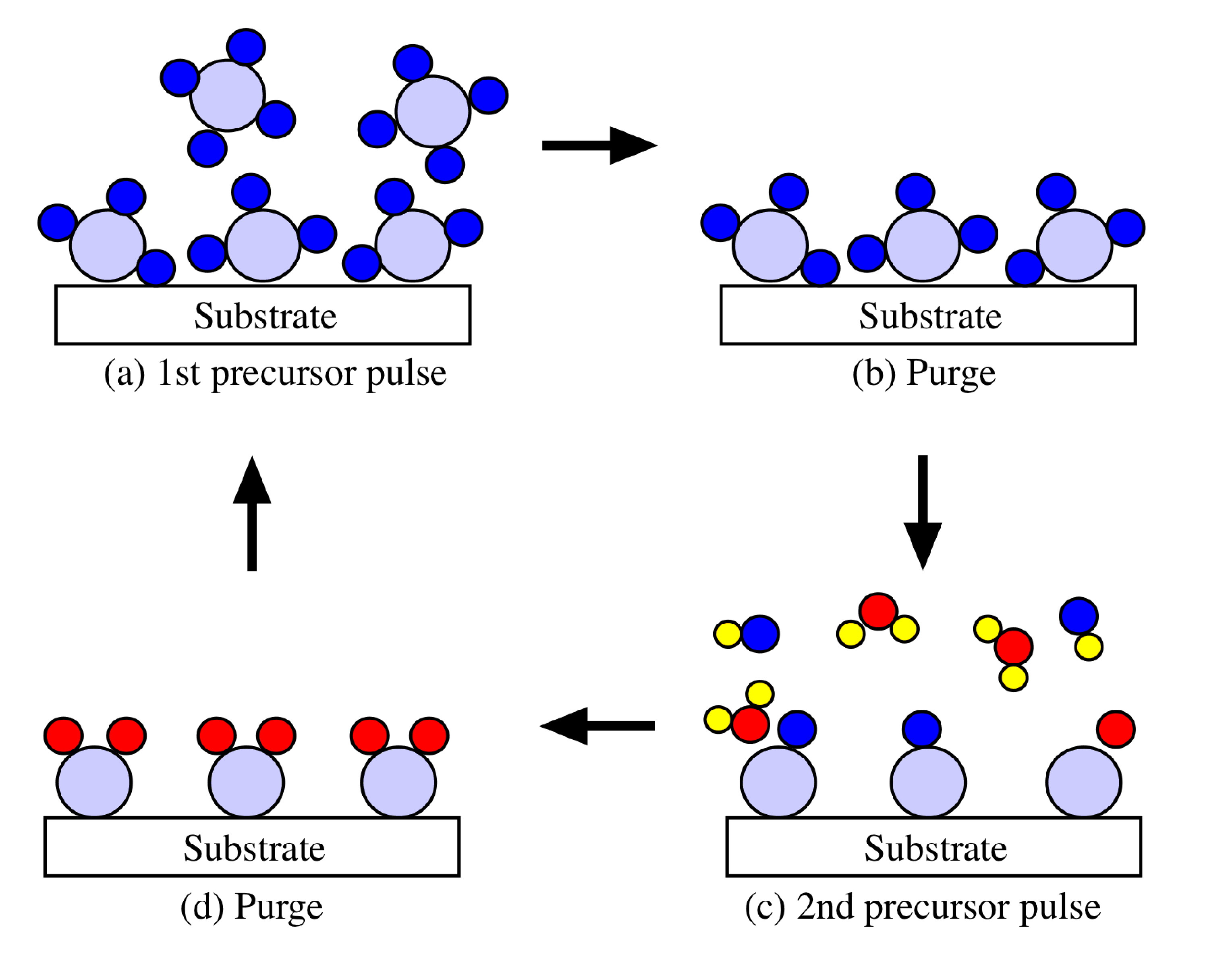
The deposition may be defined as self-limiting since one, and only one, monolayer of the reactant species remains on the surface after each exposure. In this case, one complete cycle results in the deposition of one monolayer of the compound on the substrate. Repeating this cycle leads to a controlled layer-by-layer growth. Thus the film thickness is controlled by the number of precursor cycles rather than the deposition time, as is the case for a CVD processes. This self-limiting behavior is the fundamental aspect of ALD and understanding the underlying mechanism is necessary for the future exploitation of ALD.
One basic condition for a successful ALD process is that the binding energy of a monolayer chemisorbed on a surface is higher than the binding energy of subsequent layers on top of the formed layer; the temperature of the reaction controls this. The temperature must be kept low enough to keep the monolayer on the surface until the reaction with the second reactant occurs, but high enough to re-evaporate or break the chemisorption bond. The control of a monolayer can further be influenced with the input of extra energy such as UV irradiation or laser beams. The greater the difference between the bond energy of a monolayer and the bond energies of the subsequent layers, the better the self-controlling characteristics of the process.
Basically, the ALD technique depends on the difference between chemisorption and physisorption. Physisorption involves the weak van der Waal's forces, whereas chemisorption involves the formation of relatively strong chemical bonds and requires some activation energy, therefore it may be slow and not always reversible. Above certain temperatures chemisorption dominates and it is at this temperature ALD operates best. Also, chemisorption is the reason that the process is self-controlling and insensitive to pressure and substrate changes because only one atomic or molecular layer can adsorb at the same time.
Equipment used in the ALD process may be classified in terms of their working pressure (vacuum, low pressure, atmospheric pressure), method of pulsing the precursors (moving substrate or valve sources) or according to the types of sources. Several system types are discussed.
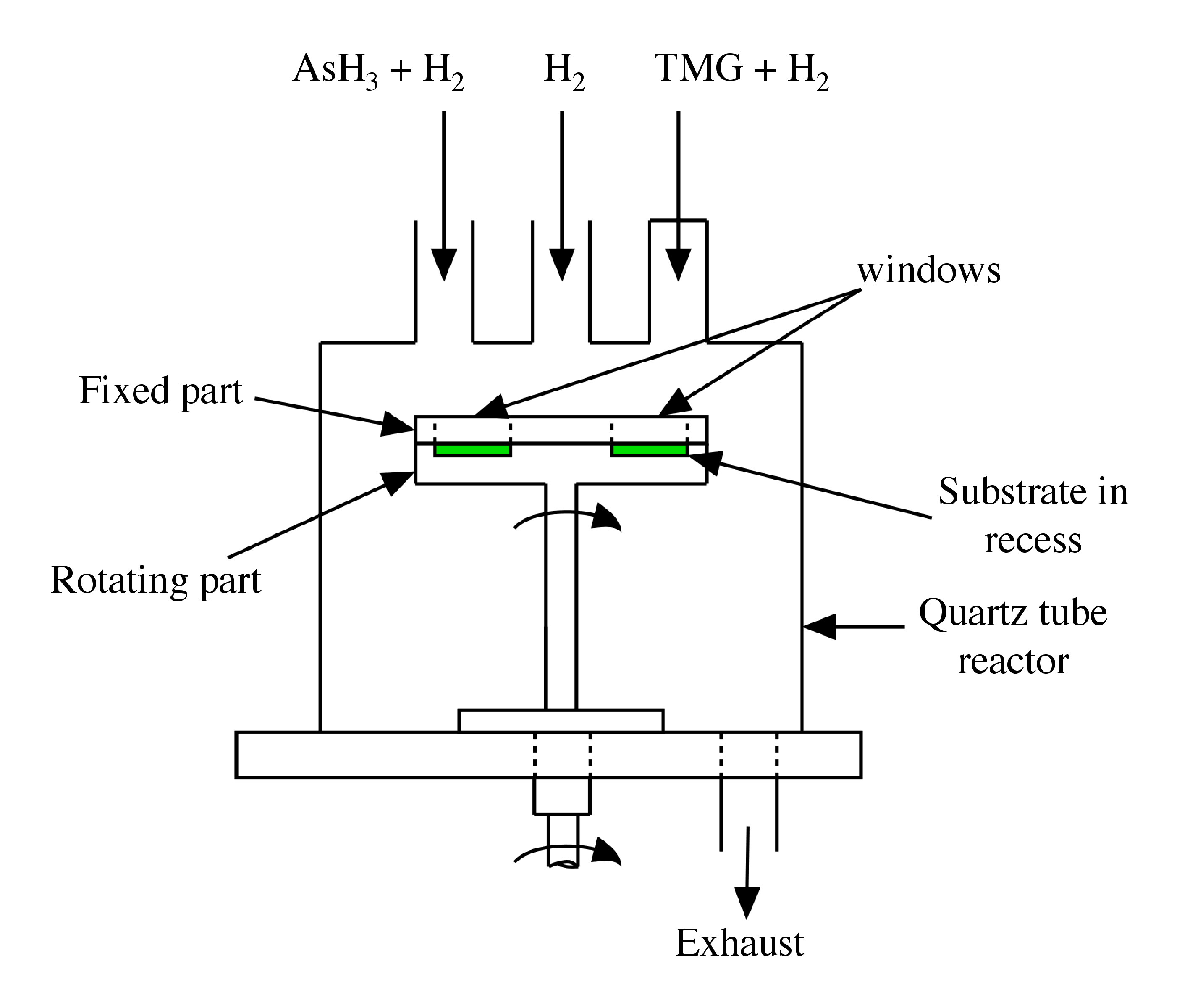
In a typical moving substrate ALD growth system ( [link] ) the substrate, located in the recess part of the susceptor, is continuously rotated and cuts through streams of the gaseous precursors, in this case, trimethylgallium [TMG, Ga(CH 3 ) 3 ] and arsine (AsH 3 ). These gaseous precursors are introduced through separate lines and the gases come in contact with the substrate only when it revolves under the inlet tube. This cycle is repeated until the required thickness of GaAs is achieved. The exposure time to each of the gas streams is about 0.3 s.

Notification Switch
Would you like to follow the 'Chemistry of electronic materials' conversation and receive update notifications?