| << Chapter < Page | Chapter >> Page > |
1.6.2. SOLAR SYSTEM MODEL OF ATOM AS PROPOSED BY ERNEST RUTHERFORD.
From 1906 to 1908 by particle collision and particle scattering, Earnest Rutherford proved that nearly 99.9% of the mass is concentrated in the nucleus of the Atom and that the diameter of the nucleus is 4 orders of magnitude smaller than the diameter of the Atom. The diameter of the Atom is of the order of Angstrom(10 -10 m) whereas the nucleus is of the order of 10 Fermi Unit where 1 Fermi Unit is 10 -15 m. Since Atoms are electrically neutral hence nucleus contains as many positive charges as are the electrons orbiting the nucleus.
From this Model, most of the Atom is vacuum. Just as the Planets are orbiting the Sun, in an analogous manner electrons are orbiting the nucleus. Furthermore the nucleus contains as many positively charged protons as the number of electrons are orbiting the nucleus.
In the particle scattering experiment a Gold or Platinum thin foil was bombarded with Alpha particles. Most of the particles passed through the foil without experiencing any deflection. It appeared as if the interatomic and most of the intra-atomic space in the foil was empty space.
Though most of the Alpha particles passed through unhindered there was a minority group of Alpha particles which did experience small deflection and there was a rare few which were completely reversed in their track. The reversal in their track was tantamount to a deflection of 180° as shown in Fig(10).
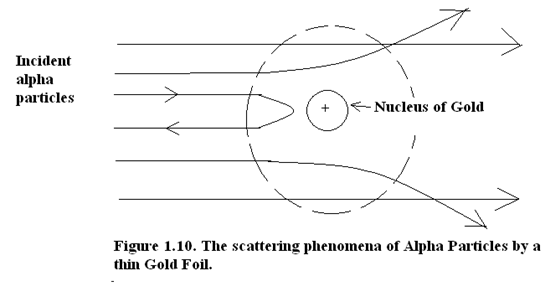
Figure 1.10. The scattering phenomena of Alpha Particles by a thin Gold Foil.
Since the electrons are 1838 th part of proton by mass therefore the orbiting electrons have no influence on incident Alpha particles which ,as will be seen ,are the nuclei of Helium Atoms. But if the incident Alpha particle experiences a direct collision with the nucleus, the former is reversed in its track. This clearly proves that Alpha particles are of comparable mass as that of the nucleus.
Here it will be appropriate to mention that Alpha , Beta and Gamma Rays accompany Radioactivity. Gamma Rays are very short energetic Electromagnetic Waves of wavelength (1/100)Å, Beta Rays are electron beam whereas Alpha Rays are the nuclei of He atoms with two protons and two neutrons.
On the basis of α particles scattering by gold foil, Rutherford proposed that most of the atom is empty space with 99.9% of the total mass concentrated at the center called nucleus and electrons are orbiting around the nucleus. The negative charge of the orbiting electrons is exactly balanced by the positive charge of the nucleus.
In 1908 Earnest Rutherford was awarded the Nobel Prize in Physics for unraveling the planetary model of the Atom.

Notification Switch
Would you like to follow the 'Solid state physics and devices-the harbinger of third wave of civilization' conversation and receive update notifications?