| << Chapter < Page | Chapter >> Page > |
SSPD_Chapter1_Part 11_Solid State of Matter_Crystalline Nature of Solid.
1.11. CRYSTALLINE STRUCTURE- SINGLE CRYSTAL, POLY CRYSTAL AND AMORPHOUS.
1.11.1. SINGLE CRYSTAL
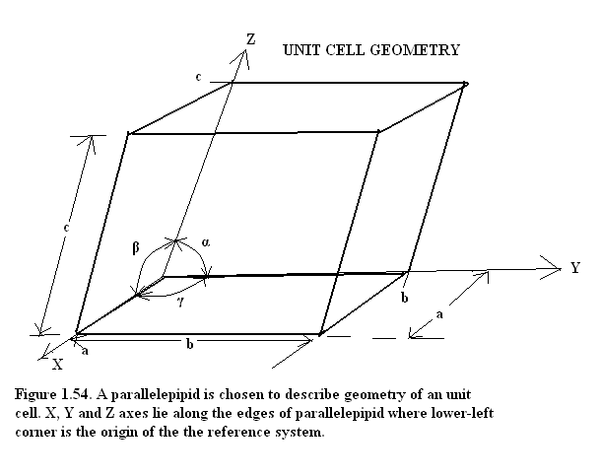
Solid State is essentially a condensed state of matter. All solids are composed of atoms, molecules or ions. The atoms are held together by ionic bond, covalent bond, van der wal’s bond or metallic bond. When a geometric configuration of atoms/molecules/ions is repeated in 3-Dimensions to generate a solid then we have a crystalline solid. The geometric configuration is called the Unit Cell. The Crystal is a periodic arrangement of these unit cells and these unit cells are the basis or the pattern of the crystal. The periodic arrangement of atoms is referred to as crystal lattice and the unit cells are referred to as the lattice centers. The unit cells are of seven basic geometric configurations as listed in Table (1.20). The unit cell have three basic reference vectors a, b, c and mutual angles of inclination α, β, γ as shown in Figure (1.54). Different combinations of three basic reference vectors a, b, c and mutual angles of inclination α, β, γ give rise to 14 different 3-D spatial configurations or space lattices called BRAVAIS LATTICE. These are 1 triclinic, 2 monoclinic, 4 rhombic, 2 tetragonal and 3 cubic. But these reduce to seven basic geometric configurations which are illustrated in Figure 1.55.
Table 1.20. The seven crystal systems.
| Serial Number | Name of the Crystal System | Length of axes. | Angle between axes. | Examples |
| 1 | Simple Cube | a=b=c | α=β=γ= 90° | Cu, NaCl |
| 2 | Tetragonal | a=b≠c | α=β=γ= 90° | SnO 2 , NiSO 4 |
| 3 | Orthorhombic | a≠b≠c | α=β=γ= 90° | KNO 3 , BaSO 4 |
| 4 | Monoclinic | a=b≠c | α=β=90 ≠ γ | Na 2 SO 4 ,FeSO 4 |
| 5 | Triclinic | a≠b≠c | α=β=90° ≠ γ | CuSO 4 , K 2 Cr 2 O |
| 6 | Trigonal(Rhombohedral) | a=b=c | α=β=γ ≠ 90° | Ca SO 4 , As |
| 7 | Hexagonal | a=b≠c | α=β=90° and γ= 120° | Zn, Cd, SiO 2 |
Cubic crystal structure, a = b = c; α = β= γ= 90º; simple, b.c.c. , f.c.c..
Tetragonal crystal structure, a = b ≠ c; α = β= γ= 90º; simple and b.c.c.
Out of 14 Bravais Lattices, 9 have unit cells with orthogonal edges.
Two monoclinic lattices (referred to orthogonal planes for clarity) a = b ≠ c ; α = β= 90º , γ; (a) simple and (b) centered;
Hexagonal Lattice . a = b ≠ c ; α = β= 90º , γ = 120º;
Triognal Lattice or Rhombohedral. It has a set of primitive vectors of equal lengths and equal angles. a = b = c; α = β= γ ≠ 90º;
Triclinic Lattice. No 90º angle. For clarity it has been referred to a cube and to orthogonal planes.
The diagrams of all these seven basic geometric configurations will uploaded as a supplement.
Figure. 1.55. The topograghy of the seven crystalline structure and 14 Bravais Lattices.
[ Semiconductor Device Electronics, Warner and Grung, Publisher: Holt, Rinehart and Winston,1991, pp.64-65 ]
Table 1.21. Comparison of cell properties of some crystal lattices.

Notification Switch
Would you like to follow the 'Solid state physics and devices-the harbinger of third wave of civilization' conversation and receive update notifications?