| << Chapter < Page | Chapter >> Page > |
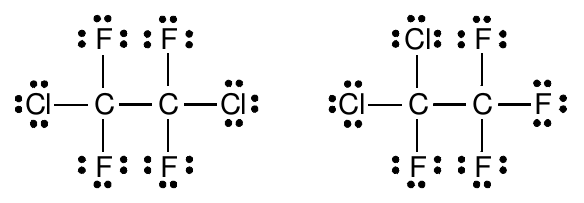
In one molecular structure, the two Cl atoms are on the same carbon atom. In the other, the two Cl atoms are separated on different carbon atoms. We might guess that this difference in arrangements does not matter, since in both cases, the F and Cl atoms are all bonded to the carbon atoms, and the carbon atoms are bonded together.
Our experimental observations prove this guess is wrong. There are two isomers of C 2 F 4 Cl 2 , one with a boiling point of 3 ˚C and a melting point of -57 ˚C and the other with a boiling point of 3.8 ˚C and a melting point of -94 ˚C. Differences in the arrangement of atoms in similar molecules clearly do matter, even if those differences don’t seem all that great.
In some cases, isomers have very obviously different structures. Let’s look at the various isomers with the molecular formula C 5 H 10 . Perhaps the simplest structure is one with a double bond between two of the carbon atoms. However, in a chain of five carbon atoms, there are two different places where the double bond might be. Each of these corresponds to a different compound:
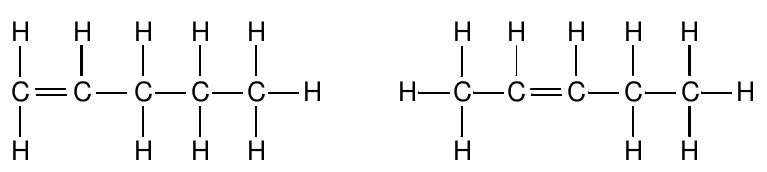
The five carbon atoms don’t have to be lined up in a single chain:
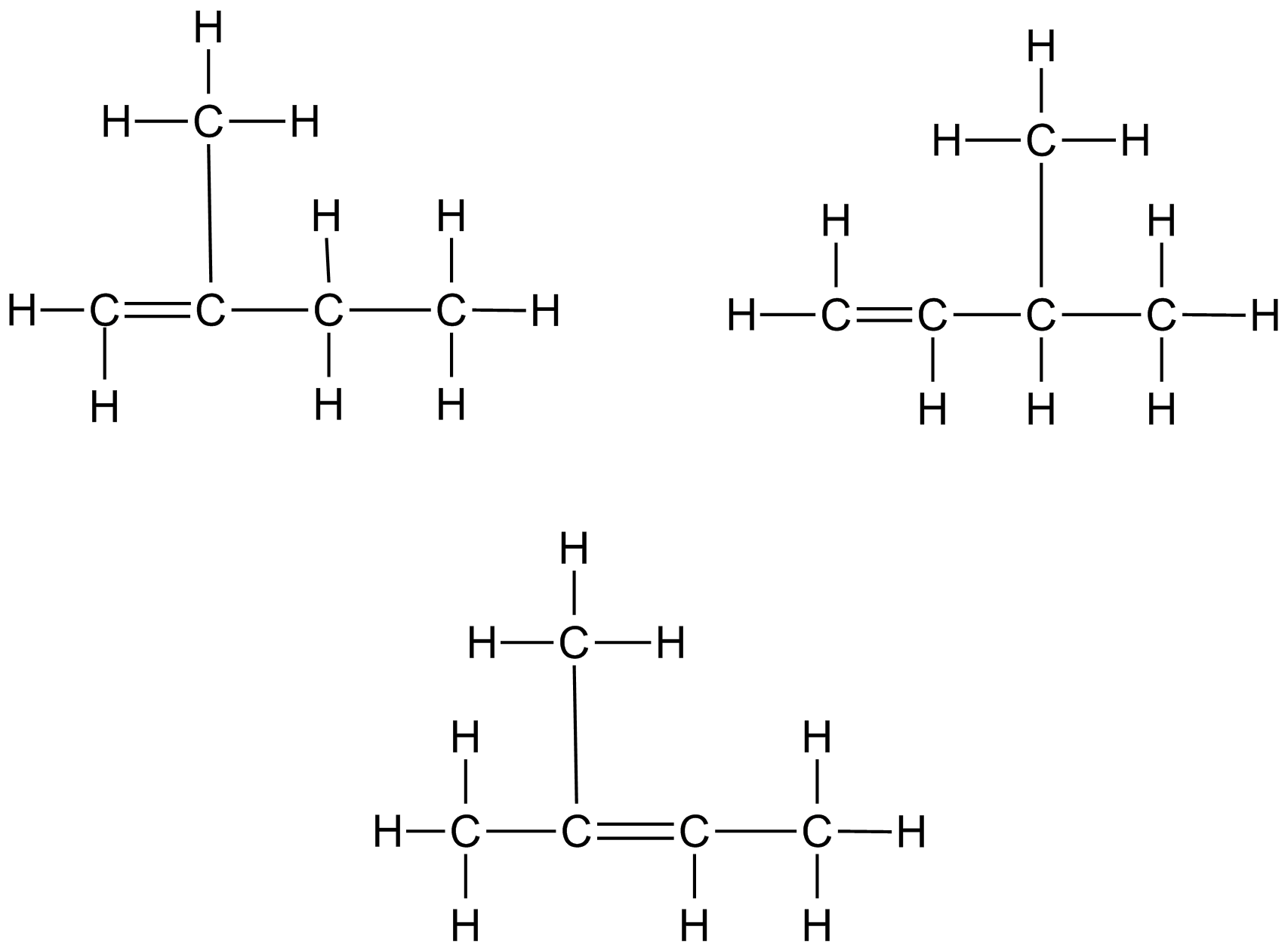
One isomer of C 5 H 10 which might not have been obvious is a structure in which the five carbon atoms form a ring:
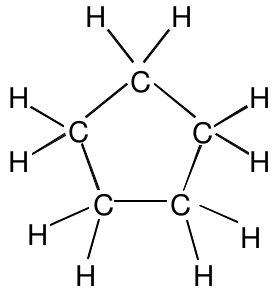
All seven of these isomers are different compounds with distinct physical and chemical properties. From these and many similar observations we can conclude as a general rule that isomers have different molecular structures, which give rise to the differing properties of the compounds.
The previous conclusion leads us to a new question: what is it about the differences in molecular structure that produces the differences in properties? This is a huge question which, in many ways, is one of the fundamental questions of the field of Organic Chemistry. Although we cannot hope to provide all the answers to this question in this one study, we can study a few examples to develop the fundamental concept.
We begin by looking for common connections amongst molecular structures and molecular properties. From the observations we just discussed, it seems that every arrangement of the atoms in a molecule produces properties which are unlike the properties of any other molecular structure. Let’s go back and examine the two isomers of C 2 H 6 O, ethanol and dimethyl ether. As noted above, ethanol is a liquid at room temperature, and is completely soluble in water. Dimethyl ether is a gas at room temperature and is less soluble in water than ethanol.
The most notable difference between the two molecular structures is that the oxygen atom is bonded to a hydrogen atom in ethanol. There are no O-H bonds in dimethyl ether. The structure C-O-H appears in a large number of molecules. And experimentally, we find that molecules with the C-O-H group have similar molecular properties to ethanol. All are liquids at room temperature and most are soluble in water. These compounds are, as a class, called alcohols. These observations lead us to conclude that common properties are due to the common C-O-H group. The C-O-H is called the “hydroxyl group”. When we find a group of atoms which gives a specific set of properties, or “function,” to molecules, we call that group of atoms a “functional group.” Using this new term, we would say that the class of molecules called alcohols contain the hydroxyl functional group.

Notification Switch
Would you like to follow the 'Concept development studies in chemistry 2012' conversation and receive update notifications?