| << Chapter < Page | Chapter >> Page > |
Molecular Shapes&Isomerism
Objectives
Before Coming to Lab . . .
Look over the following to make sure you have a basic understanding of the topics presented.
Introduction
The shape of a molecule is extremely important in determining its physical properties and reactivity. A multitude of shapes are possible, and in today’s lab, you will be looking at several.
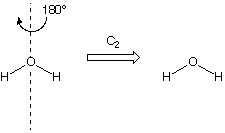
In Part 1, you will be exploring the various symmetry elements that can be present in molecules. The symmetry elements you will be looking for are mirror planes, rotation axes, and inversion centers. Being able to determine which symmetry elements are present in a molecule help in understanding its chemistry. If there is a plane present in the molecule that has the exact same arrangement of atoms on either side of the plane, then the molecule has a mirror plane ( σ ) . It is important to note that a molecule can have more than one mirror plane. Rotation axes are represented as C n (n = 1, 2, 3 . . .). The subscript indicates how many degrees of rotation (360 o /n) are needed in order to return to the same orientation of atoms with which you started. So if there is a C 2 axis, the rotation would be 180 o . An example of a molecule having a C 2 axis is H 2 O.
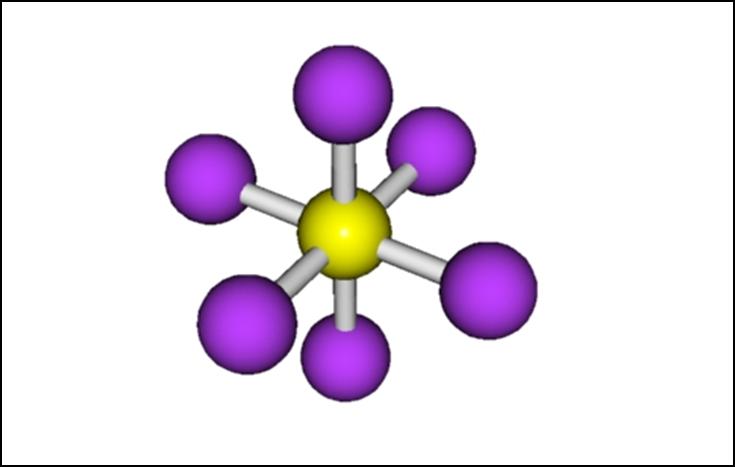
The third symmetry element is an inversion center (i) . In such molecules, starting at any position and drawing a line through the center and an equal distance to the opposite side of the molecule, you will end up at a position with an identical environment to the one you started from.
Part 2 of the lab introduces the concept of enantiomers. Enantiomers are molecules sharing the same molecular configuration, but they are non-superimposable images of each other. This concept should become clearer as you build the models for this part of the lab. Enantiomers share many of the same physical properties. The property which distinguishes them is the direction in which they rotate plane-polarized light. They will rotate the light in equal amounts but in different directions ( plane-polarized light is just light in which all wave vibrations have been filtered out except for those in one plane ). If both enantiomers are present in a 1:1 ratio, the effects of the rotation of light cancel and no net rotation is observed. Such a mixture of isomers is known as a racemic mixture or as a racemate . Because these isomers rotate plane-polarized light, they are also known as optical isomers . Compounds that form optical isomers are said to be chiral .
The chemistry of enantiomers is of great importance in the field of medicine. It has been discovered that with many drugs, one enantiomer will be biologically active while the other will be inactive or even produce undesired side effects. For this reason, it has become advantageous for pharmaceutical companies to try to synthesize the active enantiomer exclusively.

Notification Switch
Would you like to follow the 'Honors chemistry lab fall' conversation and receive update notifications?