| << Chapter < Page | Chapter >> Page > |
This sounds familiar. The rate of evaporation of molecules from the liquid phase is reduced when solute molecules are present, and now we see that the rate of freezing of molecules from the liquid phase is also reduced when solute molecules are present. Perhaps the reasons are similar. In the case of evaporation, solute molecules reduce the number of solvent molecules that are available to evaporate. In the case of freezing, solute molecules also reduce the number of solvent molecules that are available to deposit on the solid surface. This makes sense when we remember that the solute molecules cannot enter the solid phase. In essence, the solute molecules block sites on the solid surface where solvent molecules could otherwise deposit. With a lower rate of freezing than the rate of melting, the melting process wins and the solid melts.
How can we restore equilibrium? The answer is that we must lower the rate of melting. This requires that there be fewer molecules in the solid phase with sufficient energy to break free of the crystal structure. To accomplish this, the temperature must be lower. Therefore, for solid-liquid equilibrium to be restored, we have to lower the temperature, therefore lowering the freezing point.
The last example we will consider is the least obvious but is also arguably the most significant for biological systems. In this case, we will observe a solvent in equilibrium with a solution containing that solvent. This sounds tricky: how can the solvent be separated from the solution so that they can be in equilibrium with each other? Or, stated differently, how can there be two phases in such a case?
The experimental set-up may look somewhat contrived and artificial, but there are many systems in biology which have the same essential components. In Figure 5, we show flasks connected by a short glass tube. In the middle of the tube, there is a particular kind of membrane which is semi-permeable. A permeable membrane permits the passage of molecules back and forth through the membrane. A semi-permeable membrane will permit the passage only of smaller molecules; larger molecules are blocked from passing. Let’s assume that our membrane will permit the passage of the solvent, which we’ll take to be water, but will not permit the passage of a solute, which we’ll take to be glucose. Now, let’s fill both flasks up to their necks with water. Since the water can flow freely through the membrane, the level of water in both flasks will rise to the same level due to gravity. The two flasks are in equilibrium with each other, just as if the membrane wasn’t there.
Figure 5
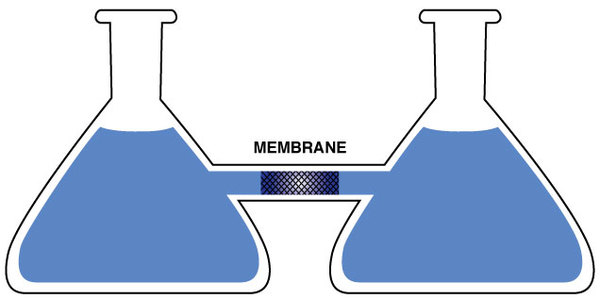
Now we will add glucose to the flask on the right. This would not seem to change anything because the glucose cannot cross the membrane from the right flask to the left flask. However, this is not what we observe experimentally. Instead, we observe that the water level begins to rise in the flask on the right while the water level falls in the flask on the left. This means that the pure water in the left flask and the solution in the right flask are no longer in equilibrium with each other. This flow continues for a while but eventually stops, meaning that the flasks eventually come to equilibrium again, but with less water in the flask on the left than there is solution in the flask on the right.

Notification Switch
Would you like to follow the 'Concept development studies in chemistry 2013' conversation and receive update notifications?