| << Chapter < Page | Chapter >> Page > |
It would be nice to add to our bonding model a way to understand or even to predict which of these types of bonding is expected for a particular solid. This would allow us to understand or predict the properties of the solid from the properties of the atoms which make it up. How shall we begin?
The most consistent trend we have seen is that bonding appears quite different for metals, non-metals, and combinations of metals and non-metals. At least from what we have observed so far, metal atoms bond to metal atoms with metallic bonding (hence the name!), metal atoms bond to non-metal atoms with ionic bonding, and non-metal atoms bond to non-metal atoms with covalent bonding. This suggests that we look at the differences between metal atoms and non-metal atoms. From our previous concept study, we know one major difference: the electronegativity of non-metals is quite high, whereas the electronegativity of metals is typically much lower.
Let’s break this down in terms of the three types of bonding. The easiest case is ionic bonding. In this case, we have combined a metal with a non-metal, like Na with Cl, so we have combined atoms with high electronegativity with atoms with low electronegativity. Apparently, atoms with these properties tend to attract each other with ionic bonding. This makes sense: with very different electronegativities, the atoms are not likely to share bonding electrons. It is likely that the very electronegative atoms will form negative ions and the weakly electronegative atoms with form positive ions, and the oppositely charged ions will attract each other. Thus, our model can be that, when a compound contains atoms with very different electronegativities, the compound is likely to be ionic bonded and have the properties of an ionic solid.
By process of elimination, the remaining types of bonding, metallic and covalent, must involve atoms with similar electronegativities. But something must distinguish these two in a way that we can predict. Metallic bonding is expected when all the atoms are metals and therefore have low electronegativity. Covalent bonding is expected when all the atoms are non-metals and therefore have relatively high electronegativity.
Here is the general summary for our model:
In the last case, there are many types of solids possible, and the properties of a covalent compound depend very much on the types of solid which is formed. To illustrate, diamond and ammonia NH 3 are both covalent compounds, but the properties of these two compounds could hardly be more different. We’ll need a more extensive model to predict what type of covalent compound will form.
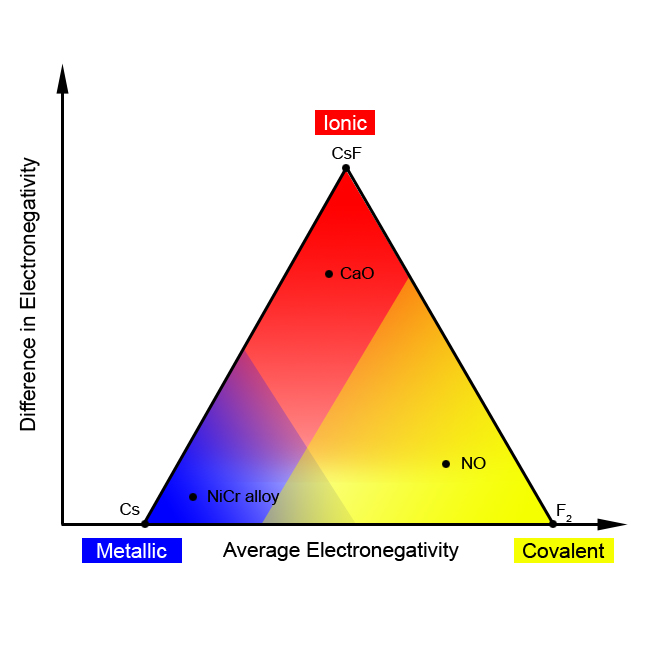
This model leads to a simple picture for a reasonable prediction of the type of bonding. We need to consider both the differences in electronegativity between the atoms in a compound as well as the actual magnitudes of the electronegativities, either high or low. This means that, at least for binary compounds (those involving only 2 elements), we can create a chart showing both the magnitude of the electronegativities of the atoms (taken as an average of the two electronegativities) and the difference between the two electronegativities. We wind up with a chart that looks like a triangle, as in [link] . In fact, this is called a “bond type triangle.” A few compounds are shown on the triangle to illustrate how this model can be used successfully to predict the type of solid from the atoms involved.

Notification Switch
Would you like to follow the 'Concept development studies in chemistry 2012' conversation and receive update notifications?