| << Chapter < Page | Chapter >> Page > |
So diamond has neither metallic bonding nor ionic bonding. But this is not a surprise, since we know already that carbon forms covalent bonds. We can recall that carbon atoms have a valence of 4 and have 4 valence electrons which they commonly share with other carbon atoms or with non-metal atoms to form covalent bonds. Since all of our observations suggest that the bonding electrons in diamond are localized, we can imagine that all of the bonding in diamond is covalent. That makes sense, based on our knowledge of the ways that carbon atoms bond with different elements.
Let’s pick one carbon atom to start with. What if that carbon atom is bonded to four other carbon atoms, satisfying the octet rule for covalent bonding? And what if each of those four carbon atoms is, in turn, bonded to three additional carbon atoms. And those twelve carbon atoms are, again in turn, bonded to three additional carbon atoms. We can build an entire “network” of carbon atoms this way, as is illustrated in [link] where the C atoms are shown with bonds connecting them.
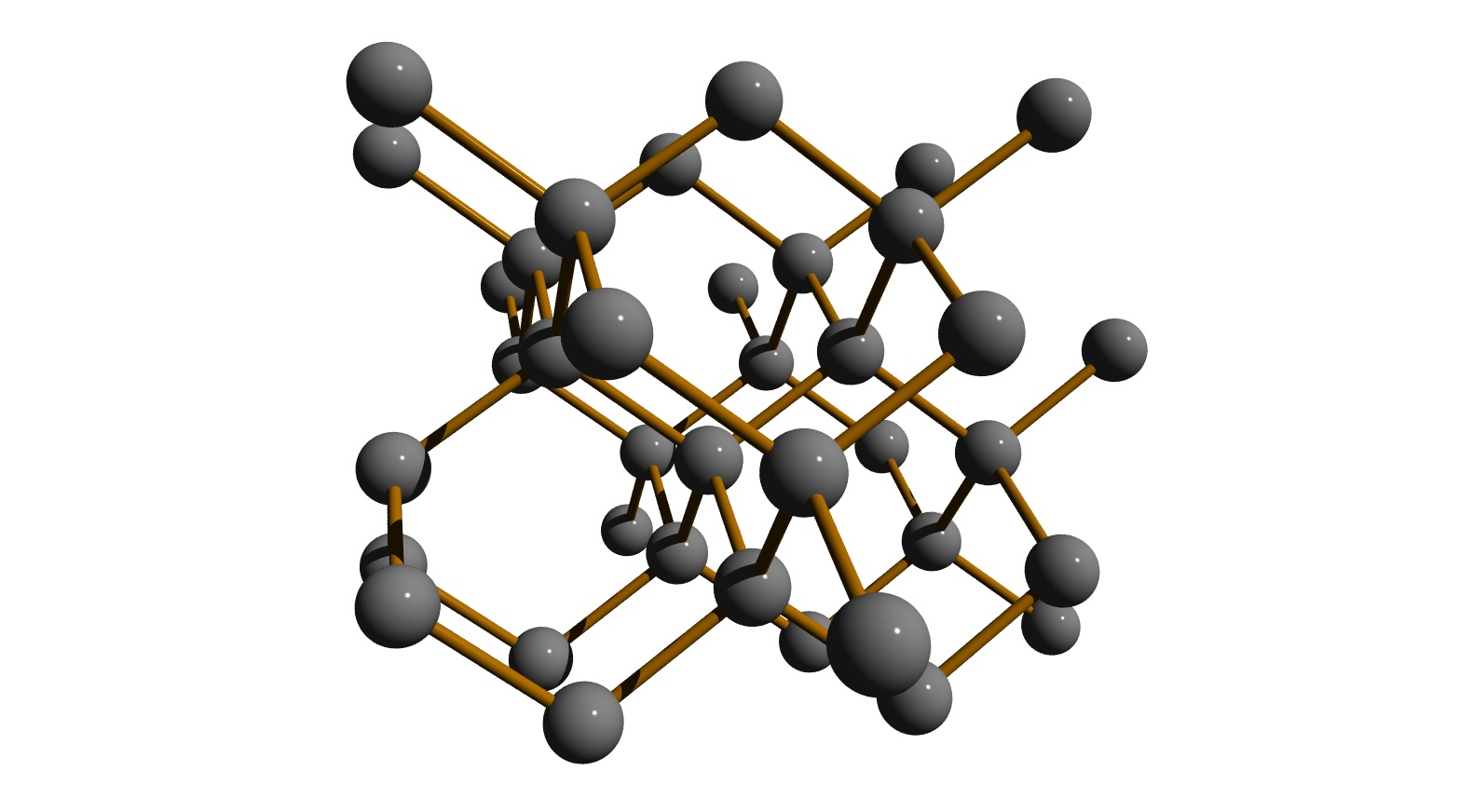
Looking closely at this, it is clear that each carbon atom has a complete octet, satisfying its valence of 4. Thinking about this more carefully, it is also clear that this network does not have to end. We could continue building this network by adding more and more carbon atoms, each bonded to four other carbon atoms, building a huge molecule large enough to hold. Or to wear on a ring!
Does the bonding model in [link] account for the properties of diamond? The electrons are all localized, so we wouldn’t expect diamond to conduct electricity. The atoms are very precisely arranged in the network and cannot be moved relative to one another without breaking some of the covalent bonds, so diamond is very hard and non-malleable. We call the bonding in diamond “network covalent”, since the bonding is all covalent and creates a network of carbon atoms.
There is an important unanswered question: why do the carbon atoms sit in the particular geometry as they do in [link] ? The answer is not obvious right now, and the question is the subject of the next concept study. For now, we’ll simply note that a carbon atom with four single bonds will arrange those bonds in the shape of a tetrahedron. When all of the carbon atoms are networked together with this geometry, we get the network in [link] .
We have now seen three types of bonding in solids. In metallic bonding, the bonding valence electrons are delocalized in an “electron sea,” allowing current to flow and permitting distortions of the arrangements of the atoms. In ionic bonding, adjacent positive metal ions and negative non-metal ions are strongly attracted to each other in an array which places positive ions next to negative ions and vice versa. This creates a hard, brittle solid, not permitting rearrangement of the ions and not allowing electron flow in an electric current. In a solid covalent network like diamond, the bonding is the more familiar covalent sharing of an electron pair so that each non-metal atom in the network satisfies its valence in agreement with the octet rule. This makes a very hard solid which is neither brittle nor malleable, and this does not allow movement of electrons in a current.

Notification Switch
Would you like to follow the 'Concept development studies in chemistry 2012' conversation and receive update notifications?