| << Chapter < Page | Chapter >> Page > |
The synthesis of WO 3-x nanorods is based on the method published by Lee et al. A slurry mixture of Me 3 NO∙2H 2 O, oleylamine and W(CO) 6 was heated up to 250 °C at a rate of 3 °C/min ( [link] ). The mixture was aged at this temperature for 3 hours before cooling down to room temperature.
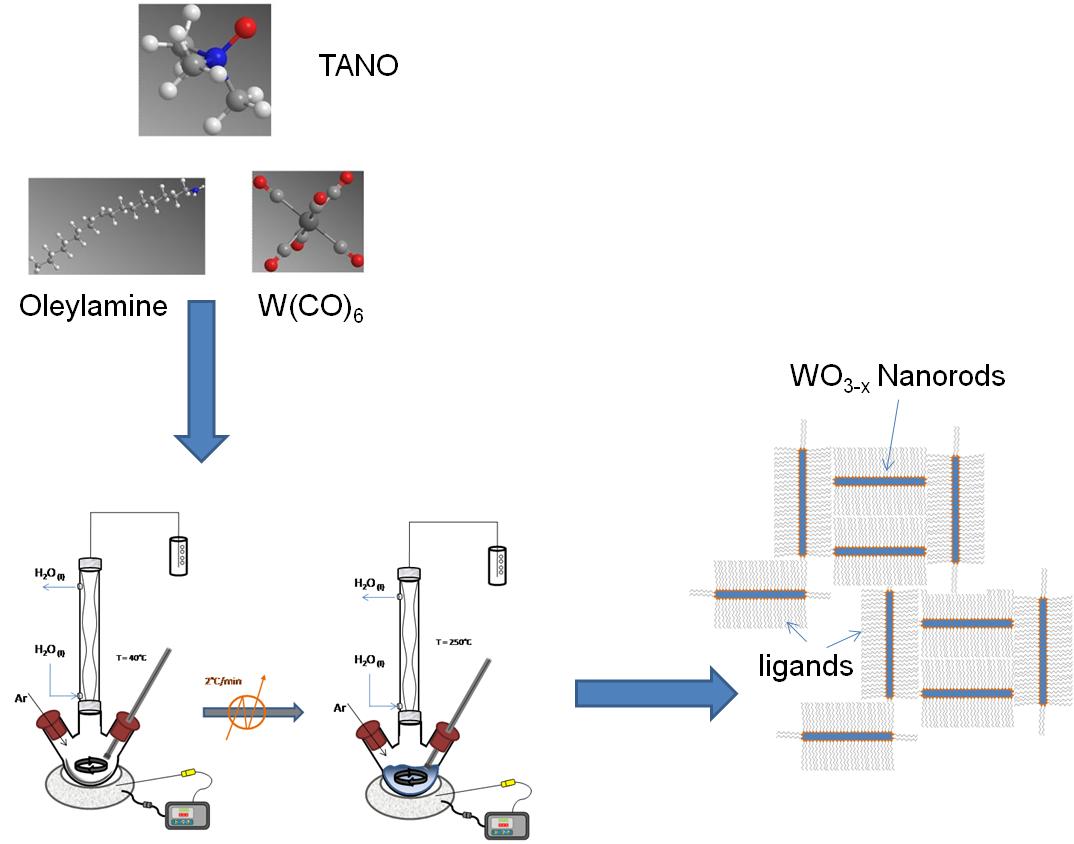
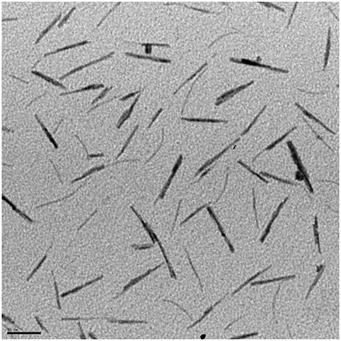
Multiple color variations were observed between 100 - 250 °C with the final product having a dark blue color. Tungsten oxide nanorods (W 18 O 49 identified by XRD) with a diameter of 7±2 nm and 50±2 nm long were acquired after centrifugation of the product solution. A TEM image of the W 18 O 49 nanorods is shown in [link] .
Thermogravimetric analysis (TGA) is a technique widely used for determining the organic and inorganic content of various materials. Its basic rule of function is the high precision measurement of weight gain/loss with increasing temperature under inert or reactive atmospheres. Each weight change corresponds to physical (crystallization, phase transformation) or chemical (oxidation, reduction, reaction) processes that take place by increasing the temperature. The sample is placed into platinum or alumina pan and along with an empty or standard pan are placed onto two high precision balances inside a high temperature oven. A method for pretreating the samples is selected and the procedure is initiated. Differential scanning calorimetry (DSC) is a technique usually accompanying TGA and is used for calculating enthalpy energy changes or heat capacity changes associated with phase transitions and/or ligand-binding energy cleavage.
In [link] the TGA/DSC plot acquired for the ligand decomposition of WO 3-x nanorods is presented. The sample was heated at constant rate under N 2 atmosphere up to 195 °C for removing moisture and then up to 700 °C for removing the oleylamine ligands. It is important to use an inert gas for performing such a study to avoid any premature oxidation and/or capping agent combustion. 26.5% of the weight loss is due to oleylamine evaporations which means about 0.004 moles per gram of sample. After isothermal heating at 700 °C for 25 min the flow was switched to air for oxidizing the ligand-free WO 3-x to WO 3 . From the DSC curve we noticed the following changes of the weight corrected heat flow:
The heat flow increase during the WO 3-x to WO 3 oxidation is proportional to the crystal phase defects (or W atoms of oxidation state +5) and can be used for performing qualitative studies between different WO x nanoparticles.
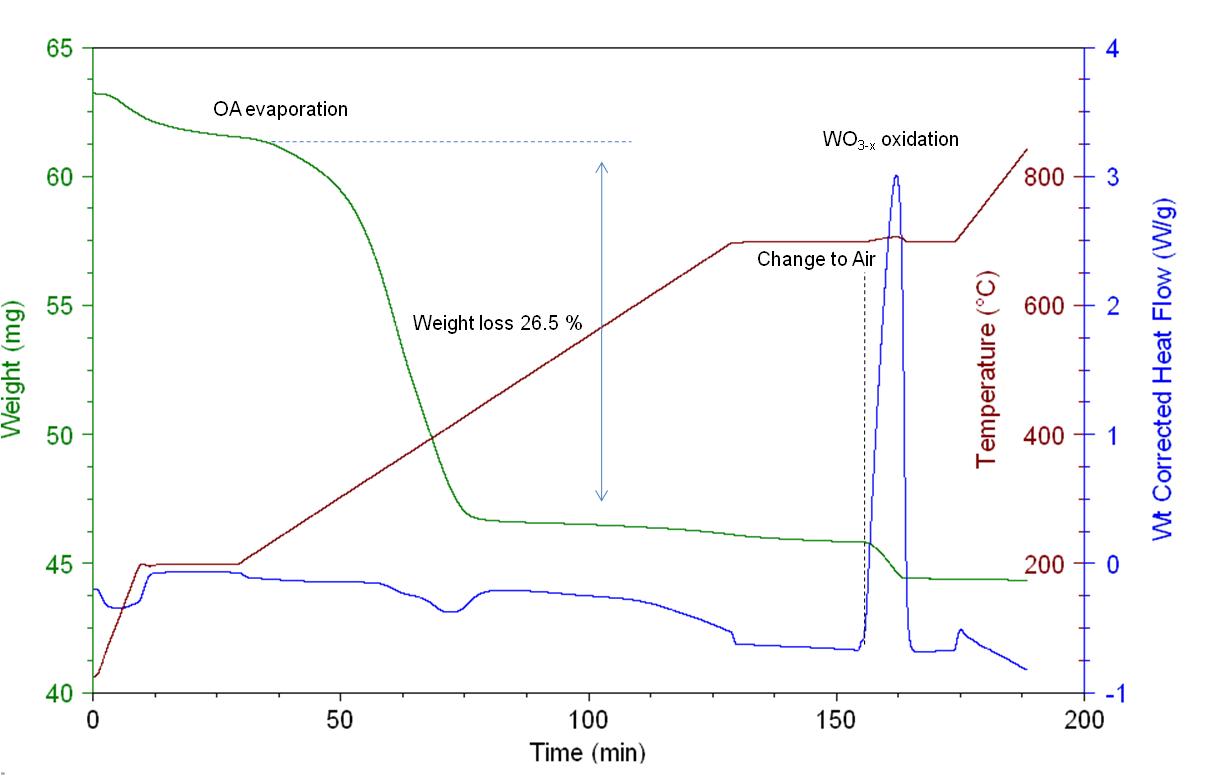
The detailed information about the procedure used to acquire the TGA/DSC plot shown in [link] is as follows.

Notification Switch
Would you like to follow the 'Nanomaterials and nanotechnology' conversation and receive update notifications?