| << Chapter < Page | Chapter >> Page > |
Another use of this light interaction can be used for imaging in biological systems, where the nanoparticles can be used as vectors to carry drugs to specific sights because of specific capping agents being used, and the internal core can be used to image the delivery and ensure the cargo is delivered correctly to the correct location in the body. One group at Rice that is working on this exciting research is the Barron lab ( (External Link) ).
Adapted from George Lisensky’s procedure that demonstrates the hydrophobicity of silver based on the Tollen’s test and the ability of self-assembly of thiol monolayers (SAM) on gold surfaces:
Essentially your TA will coat silver with a monolayer of octadecanethiol, effectively producing a non-polar surface and causing water that is dropped onto this surface to bead up.
Once your TA has placed a clean microscope slide in a Petri dish. Your TA will place 8 small drops (or 4 large drops) of a 0.5 M solution on the microscope slide ( [link] ).

Then your TA will add 25 small drops (or 12 large drops) of an active silver ion solution (made by adding concentrated ammonia drop wise to 10 mL of 0.1 M silver nitrate solution until the initial precipitate just dissolves., followed by adding 5 mL of 0.8 M KOH solution; a dark precipitate will form ( [link] ). Add more ammonia drop wise until the precipitate just redissolves. This "active silver" solution has to be used within an hour of preparation. CAUTION: To avoid the formation of explosive silver nitride, discard any remaining active solution by washing down the drain with plenty of water) to the glucose solution and gently agitate to mix the solution.
After waiting several minutes while the solution darkens and a grayish precipitate forms, a silver mirror is also forming on the slide, though it may be obscured by the precipitate c.f., Tollen’s reagent. Your TA will use water from a wash bottle to wash off the precipitate and reveal the silver mirror ( [link] ) being careful to avoid contact with the solution since it will stain their hands.
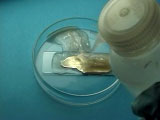
Your TA will remove the slide from the Petri dish ensuring that he/she does not touch the silver solution, and rinse the silver mirror with water. How attracted are the water drops to the surface? (Like attracts like.) Do water drops on silver spread out or bead up?
The contact angle is between the side of a drop and the slide. Is the contact angle wide (small attraction to the slide) or narrow (large attraction to the slide)?
Your TA will wait for the surface to appear dry. (For faster drying we will use a hair dryer.) Cover the silver with a few drops of a long chain alkanethiol solution , octadecanethiol, in ethanol (add a small amount of octadecanethiol, to 20 mL of ethanol. When finished, dispose of this solution by adding about 5 mL of household bleach. Let stand for several minutes then wash solution down the sink).

Notification Switch
Would you like to follow the 'Nanomaterials and nanotechnology' conversation and receive update notifications?