| << Chapter < Page | Chapter >> Page > |
Single-walled carbon nanotubes contain sp 2 carbons. Derivatives of SWNTs contain sp 3 carbons in addition. There are several factors that affect the 13 C NMR spectrum of a SWNT sample, three of which will be reviewed in this module: 13 C percentage, diameter of the nanotube, and functionalization.
For sp 2 carbons, there is a slight dependence of 13 C NMR peaks on the percentage of 13 C in the sample. Samples with lower 13 C percentage are slighted shifted downfield (higher ppm). Data are shown in [link] . Please note that these peaks are for the sp 2 carbons.
| Sample | δ (ppm) |
|---|---|
| SWNTs(100%) | 116±1 |
| SWNTs(1%) | 118±1 |
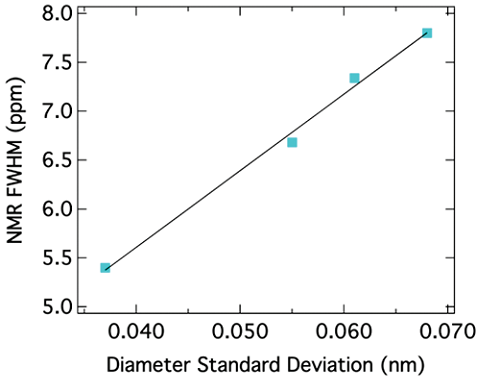
The peak position for SWNTs also depends on the diameter of the nanotubes. It has been reported that the chemical shift for sp 2 carbons decreases as the diameter of the nanotubes increases. [link] shows this correlation. Since the peak position depends on the diameter of nanotubes, the peak broadening can be related to the diameter distribution. In other words, the narrower the peak is, the smaller the diameter distribution of SWNTs is. This correlation is shown in [link] .
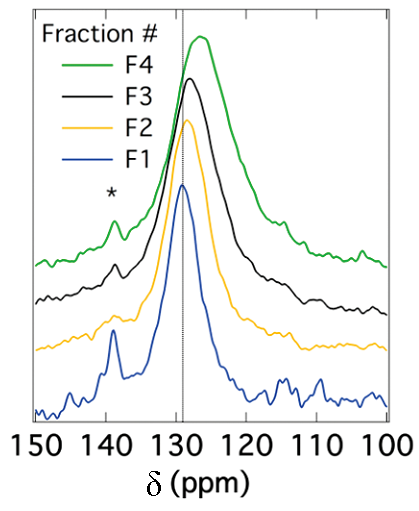
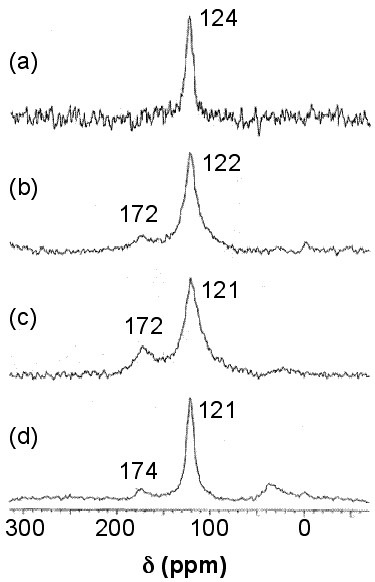
Solid stated 13 C NMR can also be used to analyze functionalized nanotubes. As a result of functionalizing SWNTs with groups containing a carbonyl group, a slight shift toward higher fields (lower ppm) for the sp 2 carbons is observed. This shift is explained by the perturbation applied to the electronic structure of the whole nanotube as a result of the modifications on only a fraction of the nanotube. At the same time, a new peak emerges at around 172 ppm, which is assigned to the carboxyl group of the substituent. The peak intensities could also be used to quantify the level of functionalization. [link] shows these changes, in which the substituents are –(CH 2 ) 3 COOH, –(CH 2 ) 2 COOH, and –(CH 2 ) 2 CONH(CH 2 ) 2 NH 2 for the spectra [link] b, [link] c, and [link] d, respectively. Note that the bond between the nanotube and the substituent is a C-C bond. Due to low sensitivity, the peak for the sp 3 carbons of the nanotube, which does not have a high quantity, is not detected. There is a small peak around 35 ppm in [link] , can be assigned to the aliphatic carbons of the substituent.

Notification Switch
Would you like to follow the 'Nanomaterials and nanotechnology' conversation and receive update notifications?