| << Chapter < Page | Chapter >> Page > |
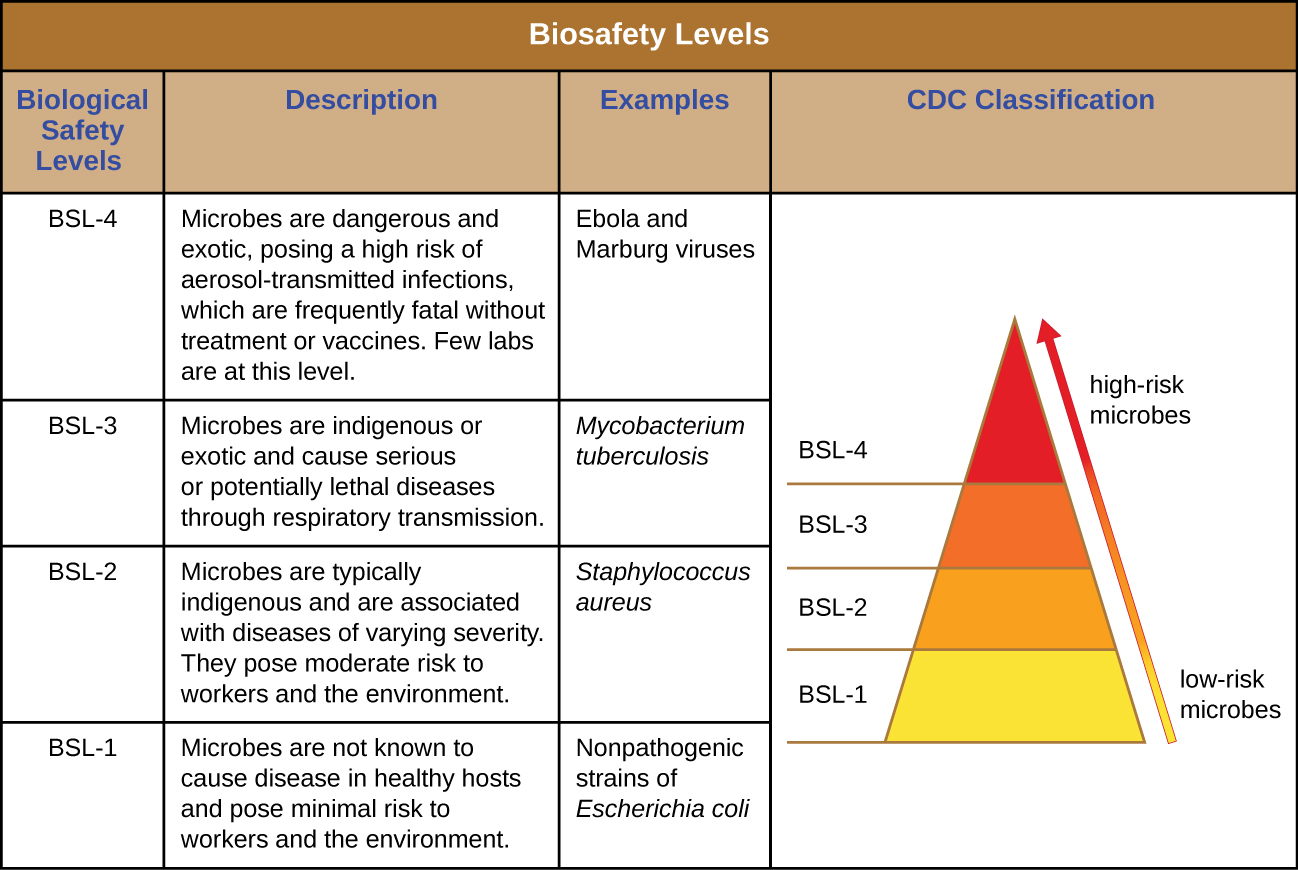
BSL-4 agents are the most dangerous and often fatal. These microbes are typically exotic, are easily transmitted by inhalation, and cause infections for which there are no treatments or vaccinations. Examples include Ebola virus and Marburg virus, both of which cause hemorrhagic fevers, and smallpox virus. There are only a small number of laboratories in the United States and around the world appropriately equipped to work with these agents. In addition to BSL-3 precautions, laboratory workers in BSL-4 facilities must also change their clothing on entering the laboratory, shower on exiting, and decontaminate all material on exiting. While working in the laboratory, they must either wear a full-body protective suit with a designated air supply or conduct all work within a biological safety cabinet with a high-efficiency particulate air (HEPA)-filtered air supply and a doubly HEPA-filtered exhaust. If wearing a suit, the air pressure within the suit must be higher than that outside the suit, so that if a leak in the suit occurs, laboratory air that may be contaminated cannot be drawn into the suit ( [link] ). The laboratory itself must be located either in a separate building or in an isolated portion of a building and have its own air supply and exhaust system, as well as its own decontamination system. The BSLs are summarized in [link] .

To learn more about the four BSLs, visit the CDC’s website.
The most extreme protocols for microbial control aim to achieve sterilization : the complete removal or killing of all vegetative cells, endospores, and viruses from the targeted item or environment. Sterilization protocols are generally reserved for laboratory, medical, manufacturing, and food industry settings, where it may be imperative for certain items to be completely free of potentially infectious agents. Sterilization can be accomplished through either physical means, such as exposure to high heat, pressure, or filtration through an appropriate filter, or by chemical means. Chemicals that can be used to achieve sterilization are called sterilant s . Sterilants effectively kill all microbes and viruses, and, with appropriate exposure time, can also kill endospores.
For many clinical purposes, aseptic technique is necessary to prevent contamination of sterile surfaces. Aseptic technique involves a combination of protocols that collectively maintain sterility, or asepsis , thus preventing contamination of the patient with microbes and infectious agents. Failure to practice aseptic technique during many types of clinical procedures may introduce microbes to the patient’s body and put the patient at risk for sepsis , a systemic inflammatory response to an infection that results in high fever, increased heart and respiratory rates, shock, and, possibly, death. Medical procedures that carry risk of contamination must be performed in a sterile field , a designated area that is kept free of all vegetative microbes, endospores, and viruses. Sterile fields are created according to protocols requiring the use of sterilized materials, such as packaging and drapings, and strict procedures for washing and application of sterilants. Other protocols are followed to maintain the sterile field while the medical procedure is being performed.

Notification Switch
Would you like to follow the 'Microbiology' conversation and receive update notifications?