| << Chapter < Page | Chapter >> Page > |
Virusoids belong to a larger group of infectious agents called satellite RNA s, which are similar pathogenic RNAs found in animals. Unlike the plant virusoids, satellite RNAs may encode for proteins; however, like plant virusoids, satellite RNAs must coinfect with a helper virus to replicate. One satellite RNA that infects humans and that has been described by some scientists as a virusoid is the hepatitis delta virus (HDV) , which, by some reports, is also called hepatitis delta virusoid. Much larger than a plant virusoid, HDV has a circular, ssRNA genome of 1,700 nucleotides and can direct the biosynthesis of HDV-associated proteins. The HDV helper virus is the hepatitis B virus (HBV) . Coinfection with HBV and HDV results in more severe pathological changes in the liver during infection, which is how HDV was first discovered.
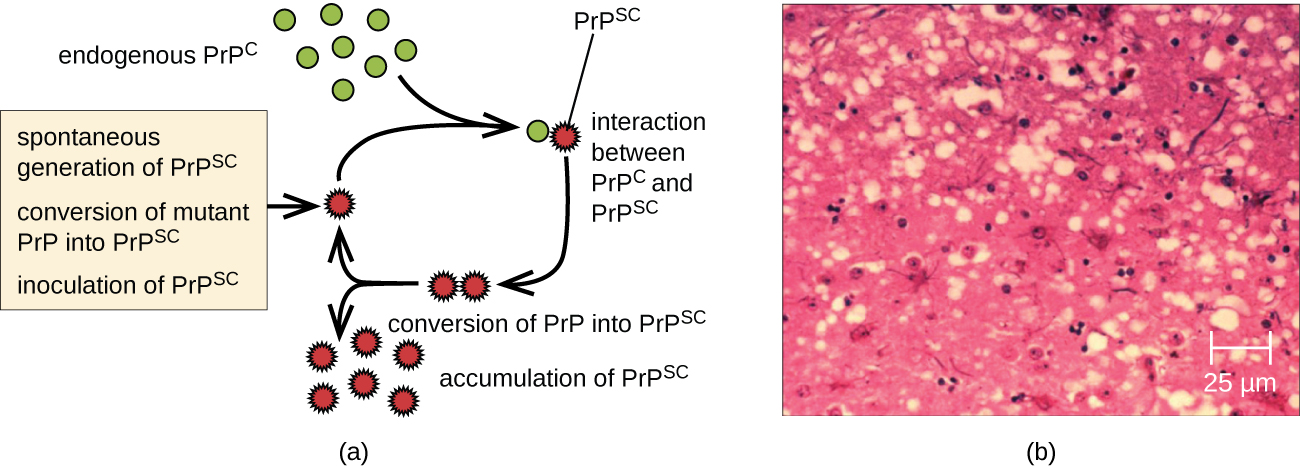
At one time, scientists believed that any infectious particle must contain DNA or RNA. Then, in 1982, Stanley Prusiner , a medical doctor studying scrapie (a fatal, degenerative disease in sheep) discovered that the disease was caused by proteinaceous infectious particles, or prion s . Because proteins are acellular and do not contain DNA or RNA, Prusiner’s findings were originally met with resistance and skepticism; however, his research was eventually validated, and he received the Nobel Prize in Physiology or Medicine in 1997.
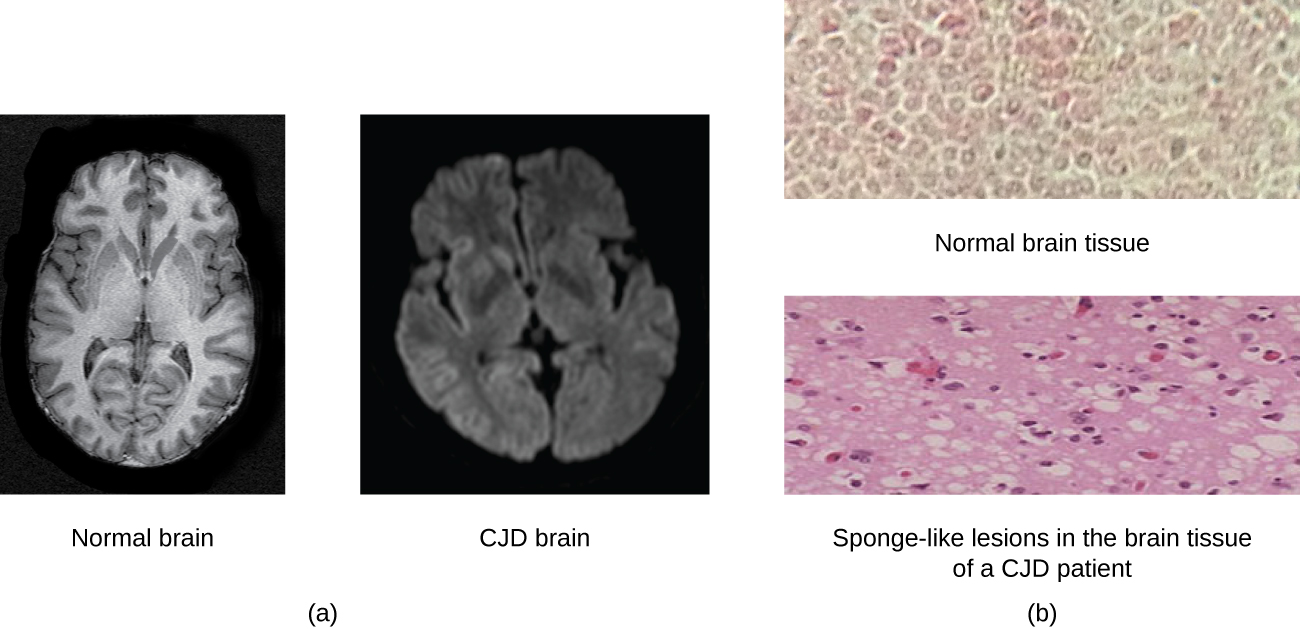
A prion is a misfolded rogue form of a normal protein (PrPc) found in the cell. This rogue prion protein (PrPsc), which may be caused by a genetic mutation or occur spontaneously, can be infectious, stimulating other endogenous normal proteins to become misfolded, forming plaques (see [link] ). Today, prions are known to cause various forms of transmissible spongiform encephalopathy (TSE) in human and animals. TSE is a rare degenerative disorder that affects the brain and nervous system. The accumulation of rogue proteins causes the brain tissue to become sponge-like, killing brain cells and forming holes in the tissue, leading to brain damage, loss of motor coordination, and dementia (see [link] ). Infected individuals are mentally impaired and become unable to move or speak. There is no cure, and the disease progresses rapidly, eventually leading to death within a few months or years.

Notification Switch
Would you like to follow the 'Microbiology' conversation and receive update notifications?