| << Chapter < Page | Chapter >> Page > |
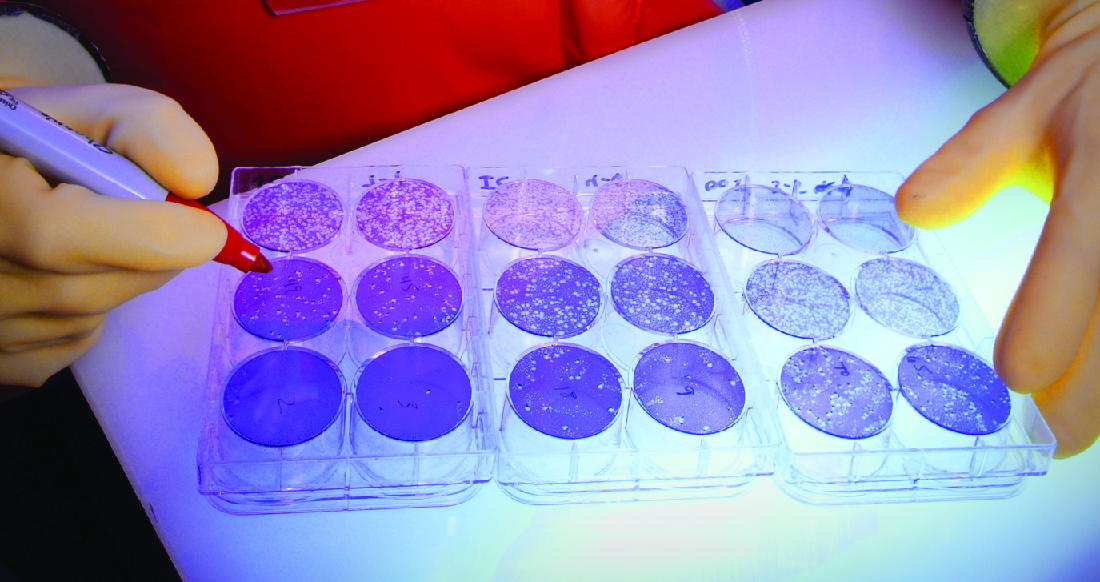
When viruses infect cells, they often cause damage ( cytopathic effects ) that may include lysis of the host cells. Cytopathic effects can be visualized by growing host cells in a petri dish, covering the cells with a thin layer of agar, and then adding virus (see Isolation, Culture, and Identification of Viruses ). The virus will diffuse very slowly through the agar. A virus will enter a host cell, proliferate (causing cell damage), be released from the dead host cell, and then move to neighboring cells. As more and more cells die, plaques of dead cells will form ( [link] ).
During the course of a viral infection, the patient will mount an antibody response to the virus, and we can quantify those antibodies using a plaque reduction assay . To perform the assay, a serial dilution is carried out on a serum sample. Each dilution is then mixed with a standardized amount of the suspect virus. Any virus-specific antibodies in the serum will neutralize some of the virus. The suspensions are then added to host cells in culture to allow any nonneutralized virus to infect the cells and form plaques after several days. The titer is defined as the reciprocal of the highest dilution showing a 50% reduction in plaques. Titer is always expressed as a whole number. For example, if a 1/64 dilution was the highest dilution to show 50% plaque reduction, then the titer is 64.
The presence of antibodies in the patient’s serum does not tell us whether the patient is currently infected or was infected in the past. Current infections can be identified by waiting two weeks and testing another serum sample. A four-fold increase in neutralizing titer in this second sample indicates a new infection.
When a patient has elevated protein levels in the blood or is losing protein in the urine, a clinician will often order a polyacrylamide gel electrophoresis ( PAGE ) assay (see Visualizing and Characterizing DNA, RNA, and Protein ). This assay compares the relative abundance of the various types of serum proteins. Abnormal protein electrophoresis patterns can be further studied using immunoelectrophoresis (IEP) . The IEP begins by running a PAGE. Antisera against selected serum proteins are added to troughs running parallel to the electrophoresis track, forming precipitin arcs similar to those seen in an Ouchterlony assay ( [link] ). This allows the identification of abnormal immunoglobulin proteins in the sample.
IEP is particularly useful in the diagnosis of multiple myeloma , a cancer of antibody-secreting cells. Patients with multiple myeloma cannot produce healthy antibodies; instead they produce abnormal antibodies that are monoclonal proteins (M proteins). Thus, patients with multiple myeloma will present with elevated serum protein levels that show a distinct band in the gamma globulin region of a protein electrophoresis gel and a sharp spike (in M protein) on the densitometer scan rather than the normal broad smear ( [link] ). When antibodies against the various types of antibody heavy and light chains are used to form precipitin arcs, the M protein will cause distinctly skewed arcs against one class of heavy chain and one class of light chain as seen in [link] .

Notification Switch
Would you like to follow the 'Microbiology' conversation and receive update notifications?