| << Chapter < Page | Chapter >> Page > |
All events had a spiritual connotation. Sickness, for example, might be a sign that a person had sinned, while crop failure could result from the villagers’ not saying their prayers. Penitents confessed their sins to the priest, who absolved them and assigned them penance to atone for their acts and save themselves from eternal damnation. Thus the parish priest held enormous power over the lives of his parishioners.
Ultimately, the pope decided all matters of theology, interpreting the will of God to the people, but he also had authority over temporal matters. Because the Church had the ability to excommunicate people, or send a soul to hell forever, even monarchs feared to challenge its power. It was also the seat of all knowledge. Latin, the language of the Church, served as a unifying factor for a continent of isolated regions, each with its own dialect; in the early Middle Ages, nations as we know them today did not yet exist. The mostly illiterate serfs were thus dependent on those literate priests to read and interpret the Bible, the word of God, for them.
The year 622 brought a new challenge to Christendom. Near Mecca, Saudi Arabia, a prophet named Muhammad received a revelation that became a cornerstone of the Islamic faith. The Koran , which Muhammad wrote in Arabic, contained his message, affirming monotheism but identifying Christ not as God but as a prophet like Moses, Abraham, David, and Muhammad. Following Muhammad’s death in 632, Islam spread by both conversion and military conquest across the Middle East and Asia Minor to India and northern Africa, crossing the Straits of Gibraltar into Spain in the year 711 ( [link] ).
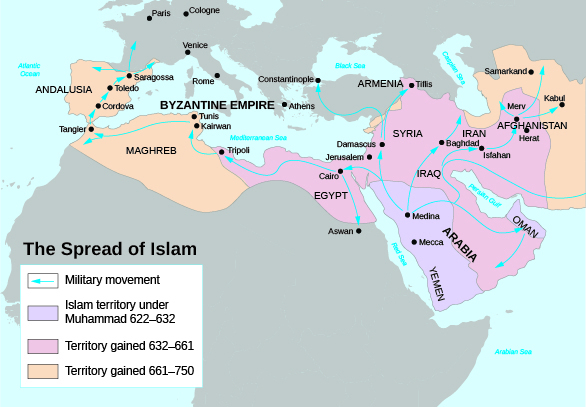
The Islamic conquest of Europe continued until 732. Then, at the Battle of Tours (in modern France), Charles Martel, nicknamed the Hammer, led a Christian force in defeating the army of Abdul Rahman al-Ghafiqi. Muslims, however, retained control of much of Spain, where Córdoba, known for leather and wool production, became a major center of learning and trade. By the eleventh century, a major Christian holy war called the Reconquista , or reconquest, had begun to slowly push the Muslims from Spain. This drive was actually an extension of the earlier military conflict between Christians and Muslims for domination of the Holy Land (the Biblical region of Palestine), known as the Crusades .
Visit EyeWitness to History to read a personal account of the Crusades.
The city of Jerusalem is a holy site for Jews, Christians, and Muslims. It was here King Solomon built the Temple in the tenth century BCE. It was here the Romans crucified Jesus in 33 CE, and from here, Christians maintain, he ascended into heaven, promising to return. From here, Muslims believe, Muhammad traveled to heaven in 621 to receive instructions about prayer. Thus claims on the area go deep, and emotions about it run high, among followers of all three faiths. Evidence exists that the three religions lived in harmony for centuries. In 1095, however, European Christians decided not only to retake the holy city from the Muslim rulers but also to conquer what they called the Holy Lands, an area that extended from modern-day Turkey in the north along the Mediterranean coast to the Sinai Peninsula and that was also held by Muslims. The Crusades had begun.

Notification Switch
Would you like to follow the 'U.s. history' conversation and receive update notifications?