| << Chapter < Page | Chapter >> Page > |

[link] shows a schematic of the polishing process. Polishing may be conducted on single wafers or as a batch process depending on the equipment employed. Single wafer polishing is preferred for larger wafers and allows for better surface flatness. In both processes, wafers are mounted onto a fixture, by either wax or a composite Felx-Mount™, and pressed against the polishing pad. The polishing pad is usually made from an artificial fabric such as polyester felt-polyurethane laminate. Polishing is accomplished by a mechano-chemical process in which aqueous polishing slurry is dripped onto the polishing pad, see [link] . The polishing slurry performs both a chemical and mechanical process, and consists of fine silica (SiO 2 ) particles (100 Å diameter) and an oxidizing agent. Aqueous sodium hydroxide (NaOH) is used for Si, while aqueous sodium chlorate (NaOCl) is preferred for GaAs. Suspending agents are usually added to prevent settling of the silica particles. Under the heat caused by the friction of the wafer on the polishing pad the wafer surface is oxidized, which is the chemical step, while in the mechanical step the silica particles in the slurry abrade the oxidized surface away.
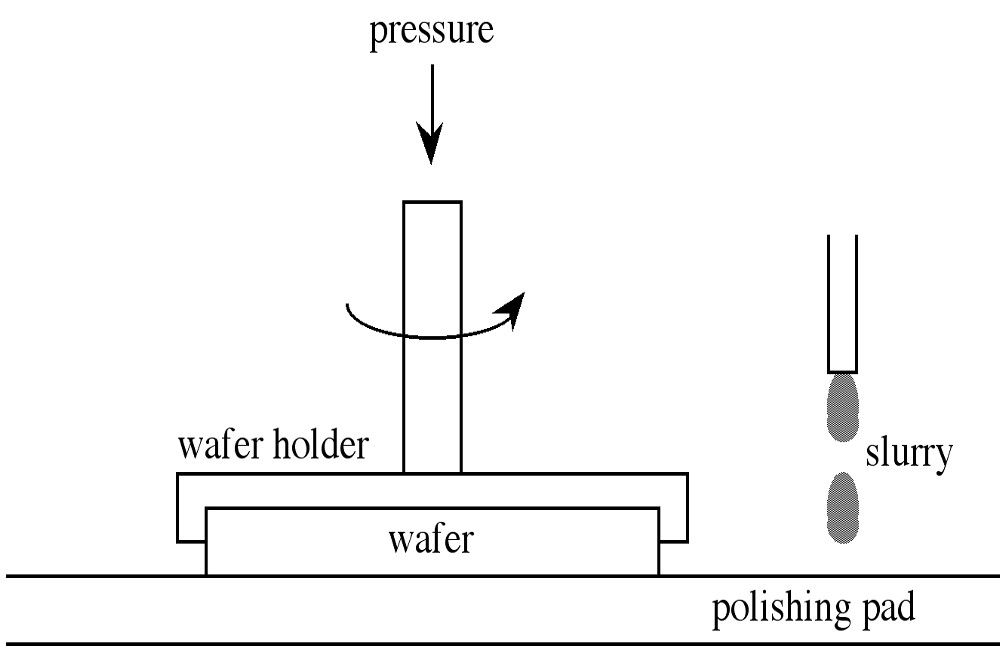
In order to achieve a reasonable rate of removal of the relief and damaged layers and still obtain the highest quality surface, the polishing is done in two steps, stock removal and haze removal. The former is carried out with a higher concentration slurry and may proceed for about 30 minutes at a removal rate of 1 μm/min. Haze removal is performed with a very dilute slurry, a softer pad with a reaction time of about 5 to 10 minutes, during which the total amount of material removed is only about 1 μm. Due to the active chemical reaction between the wafer and the polishing agent, the wafers must be rinsed in deionized water immediately after polishing to prevent haze or stains from reforming.
There are many variables that will influence the rate and quality of polishing. High pressure results in a higher polishing rate, but excessive pressure may cause non-uniform polishing, excessive heat generation and fast pad wear. The rate of polishing is increased with higher temperatures but this may also lead to haze formation. High wheel speeds accelerate the polishing rate but can raise the temperature and also results in problems in maintaining a uniform flow of slurry across the pad. Dense slurry concentrations increase the polishing rate but are more costly. The pH of the slurry solution can also affect the polishing rate, for example the polishing rate of Si gradually increases with increased pH (higher basicity) until a pH of about 12 where a dramatic decrease is observed. In general, the optimum polishing process for a given facility depends largely upon the interplay of product specification, yields, cost, and quality considerations and must be developed uniquely. The wafer polishing process does not improve the wafer flatness and, at best, polishing will not degrade the wafer flatness achieved in the lapping operation.

Notification Switch
Would you like to follow the 'Chemistry of electronic materials' conversation and receive update notifications?