| << Chapter < Page | Chapter >> Page > |
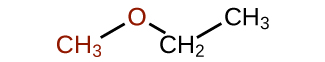
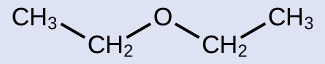
Common: The groups attached to the oxygen atom are both ethyl groups, so the common name would be diethyl ether.
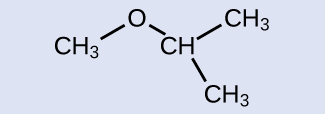
IUPAC: 2-methoxypropane; common: isopropylmethyl ether
Ethers can be obtained from alcohols by the elimination of a molecule of water from two molecules of the alcohol. For example, when ethanol is treated with a limited amount of sulfuric acid and heated to 140 °C, diethyl ether and water are formed:
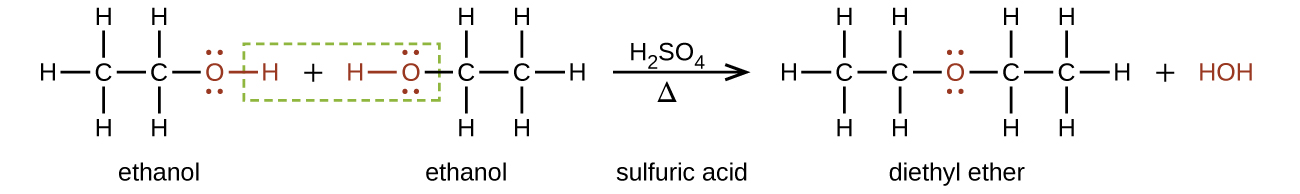
In the general formula for ethers, R— O —R, the hydrocarbon groups (R) may be the same or different. Diethyl ether, the most widely used compound of this class, is a colorless, volatile liquid that is highly flammable. It was first used in 1846 as an anesthetic, but better anesthetics have now largely taken its place. Diethyl ether and other ethers are presently used primarily as solvents for gums, fats, waxes, and resins. Tertiary -butyl methyl ether, C 4 H 9 OCH 3 (abbreviated MTBE—italicized portions of names are not counted when ranking the groups alphabetically—so butyl comes before methyl in the common name), is used as an additive for gasoline. MTBE belongs to a group of chemicals known as oxygenates due to their capacity to increase the oxygen content of gasoline.
Want more practice naming ethers? This brief video review summarizes the nomenclature for ethers.
Carbohydrates are large biomolecules made up of carbon, hydrogen, and oxygen. The dietary forms of carbohydrates are foods rich in these types of molecules, like pastas, bread, and candy. The name “carbohydrate” comes from the formula of the molecules, which can be described by the general formula C m (H 2 O) n , which shows that they are in a sense “carbon and water” or “hydrates of carbon.” In many cases, m and n have the same value, but they can be different. The smaller carbohydrates are generally referred to as “sugars,” the biochemical term for this group of molecules is “saccharide” from the Greek word for sugar ( [link] ). Depending on the number of sugar units joined together, they may be classified as monosaccharides (one sugar unit), disaccharides (two sugar units), oligosaccharides (a few sugars), or polysaccharides (the polymeric version of sugars—polymers were described in the feature box earlier in this chapter on recycling plastics). The scientific names of sugars can be recognized by the suffix -ose at the end of the name (for instance, fruit sugar is a monosaccharide called “fructose” and milk sugar is a disaccharide called lactose composed of two monosaccharides, glucose and galactose, connected together). Sugars contain some of the functional groups we have discussed: Note the alcohol groups present in the structures and how monosaccharide units are linked to form a disaccharide by formation of an ether.
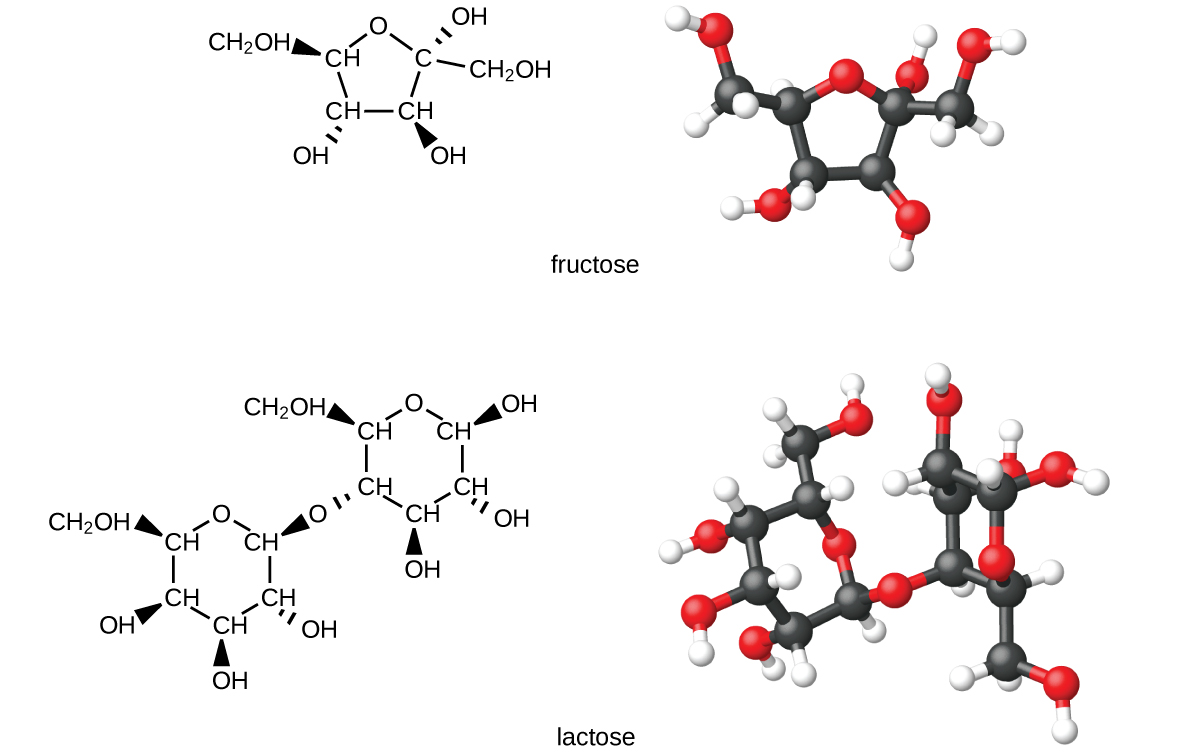
Organisms use carbohydrates for a variety of functions. Carbohydrates can store energy, such as the polysaccharides glycogen in animals or starch in plants. They also provide structural support, such as the polysaccharide cellulose in plants and the modified polysaccharide chitin in fungi and animals. The sugars ribose and deoxyribose are components of the backbones of RNA and DNA, respectively. Other sugars play key roles in the function of the immune system, in cell-cell recognition, and in many other biological roles.
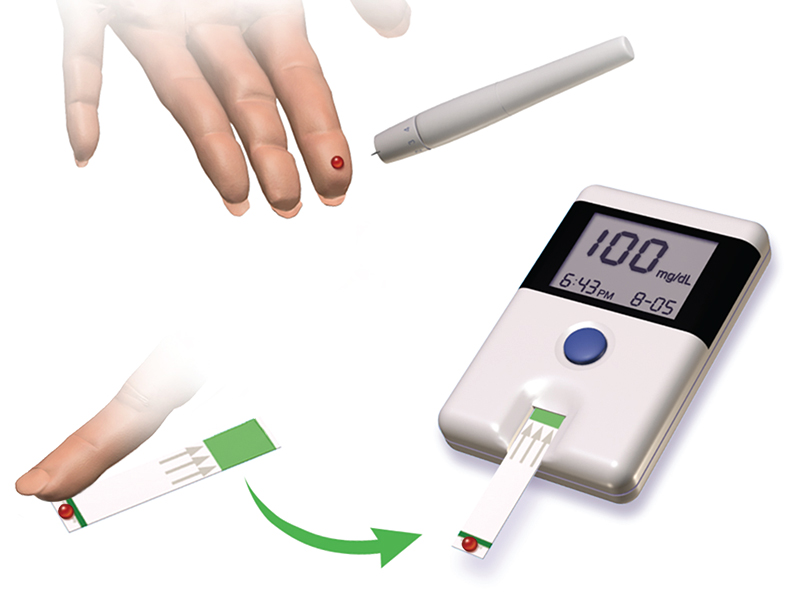
Diabetes is a group of metabolic diseases in which a person has a high sugar concentration in their blood ( [link] ). Diabetes may be caused by insufficient insulin production by the pancreas or by the body’s cells not responding properly to the insulin that is produced. In a healthy person, insulin is produced when it is needed and functions to transport glucose from the blood into the cells where it can be used for energy. The long-term complications of diabetes can include loss of eyesight, heart disease, and kidney failure.
In 2013, it was estimated that approximately 3.3% of the world’s population (~380 million people) suffered from diabetes, resulting in over a million deaths annually. Prevention involves eating a healthy diet, getting plenty of exercise, and maintaining a normal body weight. Treatment involves all of these lifestyle practices and may require injections of insulin.
Many organic compounds that are not hydrocarbons can be thought of as derivatives of hydrocarbons. A hydrocarbon derivative can be formed by replacing one or more hydrogen atoms of a hydrocarbon by a functional group, which contains at least one atom of an element other than carbon or hydrogen. The properties of hydrocarbon derivatives are determined largely by the functional group. The –OH group is the functional group of an alcohol. The –R–O–R– group is the functional group of an ether.
Why do the compounds hexane, hexanol, and hexene have such similar names?
Write condensed formulas and provide IUPAC names for the following compounds:
(a) ethyl alcohol (in beverages)
(b) methyl alcohol (used as a solvent, for example, in shellac)
(c) ethylene glycol (antifreeze)
(d) isopropyl alcohol (used in rubbing alcohol)
(e) glycerine
(a) ethyl alcohol, ethanol: CH 3 CH 2 OH; (b) methyl alcohol, methanol: CH 3 OH; (c) ethylene glycol, ethanediol: HOCH 2 CH 2 OH; (d) isopropyl alcohol, 2-propanol: CH 3 CH(OH)CH 3 ; (e) glycerine, l,2,3-trihydroxypropane: HOCH 2 CH(OH)CH 2 OH
Give the complete IUPAC name for each of the following compounds:
(a)
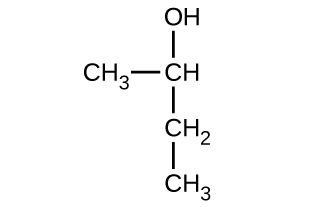
(b)
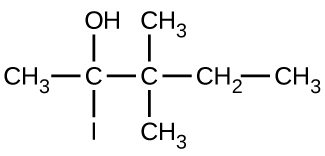
(c)
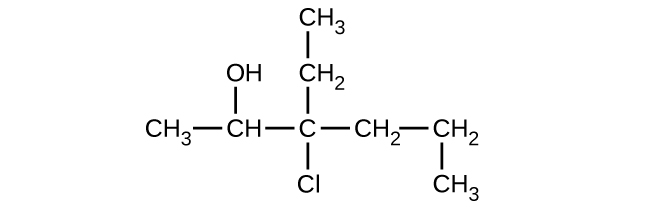
Give the complete IUPAC name and the common name for each of the following compounds:
(a)
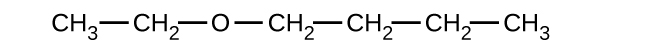
(b)
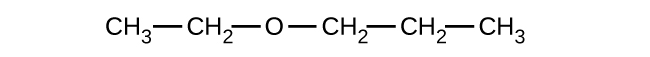
(c)
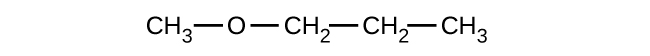
(a) 1-ethoxybutane, butyl ethyl ether; (b) 1-ethoxypropane, ethyl propyl ether; (c) 1-methoxypropane, methyl propyl ether
Write the condensed structures of both isomers with the formula C 2 H 6 O. Label the functional group of each isomer.
Write the condensed structures of all isomers with the formula C 2 H 6 O 2 . Label the functional group (or groups) of each isomer.
HOCH 2 CH 2 OH, two alcohol groups; CH 3 OCH 2 OH, ether and alcohol groups
Draw the condensed formulas for each of the following compounds:
(a) dipropyl ether
(b) 2,2-dimethyl-3-hexanol
(c) 2-ethoxybutane
MTBE, Methyl tert -butyl ether, CH 3 OC(CH 3 ) 3 , is used as an oxygen source in oxygenated gasolines. MTBE is manufactured by reacting 2-methylpropene with methanol.
(a) Using Lewis structures, write the chemical equation representing the reaction.
(b) What volume of methanol, density 0.7915 g/mL, is required to produce exactly 1000 kg of MTBE, assuming a 100% yield?
(a)
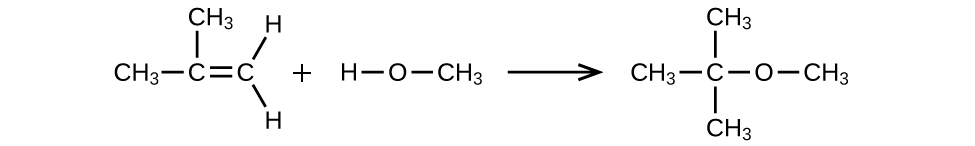 ;
;
(b) 4.593
10
2 L
Write two complete balanced equations for each of the following reactions, one using condensed formulas and one using Lewis structures.
(a) propanol is converted to dipropyl ether
(b) propene is treated with water in dilute acid.
Write two complete balanced equations for each of the following reactions, one using condensed formulas and one using Lewis structures.
(a) 2-butene is treated with water in dilute acid
(b) ethanol is dehydrated to yield ethene
(a)
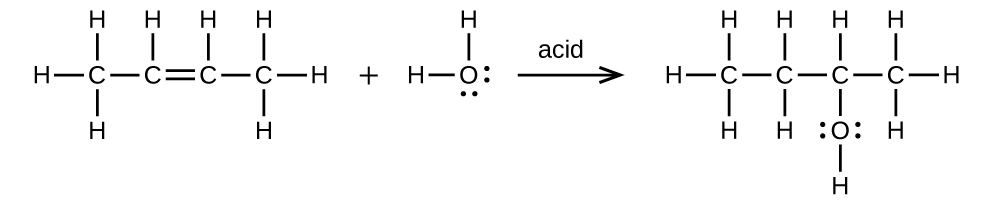 ;
;
(b)
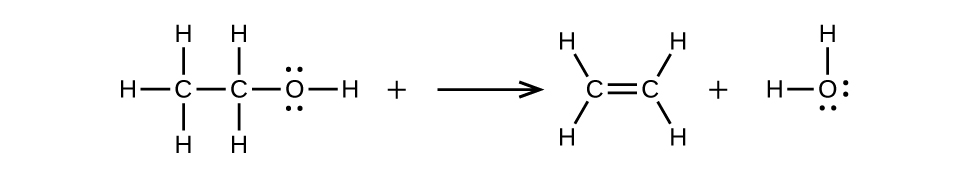

Notification Switch
Would you like to follow the 'Chemistry' conversation and receive update notifications?