| << Chapter < Page | Chapter >> Page > |
Schiff-base complexes include Cu(acim) 2 , Cu(acen) and Cu(nona-F) 2 . The first two of these can be prepared by mixing Cu(NH 3 ) 4 2+ (aq) with the pure ligand and by adding freshly prepared solid Cu(OH) 2 to a solution of the ligand in acetone. The synthesis of Cu(nona-F) 2 , on the other hand, involved two important developments: the introduction of the silyl enol ether route to the ligand and its conversion in-situ into the desired precursor. The new approach to the ligand was required because, in contrast to non-fluorinated b-diketonates, H(hfac) reacts with amines to produce salts.
Starting from the experimental results, a list of possible steps for Cu CVD via H 2 reduction of Cu(II) compounds would include the followings, where removal of adsorbed ligand from the surface is believed to be the rate limiting step:
Cu(II)L 2 (g) → Cu(s) + 2 L . (ads)
H 2 (g) → 2 H . (ads)
L . (ads) + H . (ads) → HL(g)
where L represents any of the singly charged β-diketonate or β-ketoiminate ligands described before. This mechanism gives a clear explanation of the importance of hydrogen being present: in the absence of hydrogen, HL cannot desorb cleanly into the gas phase and ligand will tend to decompose on the surface, resulting in impurity incorporation into the growing film. The mechanism is also supported by the observation that the deposition reaction is enhanced by the addition of alcohol containing β-hydrogen to the reaction mixture.
More recently, the focus has shifted to Cu(I) compounds including Cu(I) cyclopentadienyls and Cu(I) β-diketonate. The Cu(I) β-diketonate in particular show great promise as Cu CVD precursors and have superseded the Cu(II) β-diketonate as the best family of precursors currently available.
The Cu(I) compounds that have been investigated are described in [link] . These species can be broadly divided into two classes, CuX and XCuL n , where X is a uninegative ligand and L is a neutral Lewis base electron pair donor. The XCuL n class can be further subdivided according to the nature of X and L.
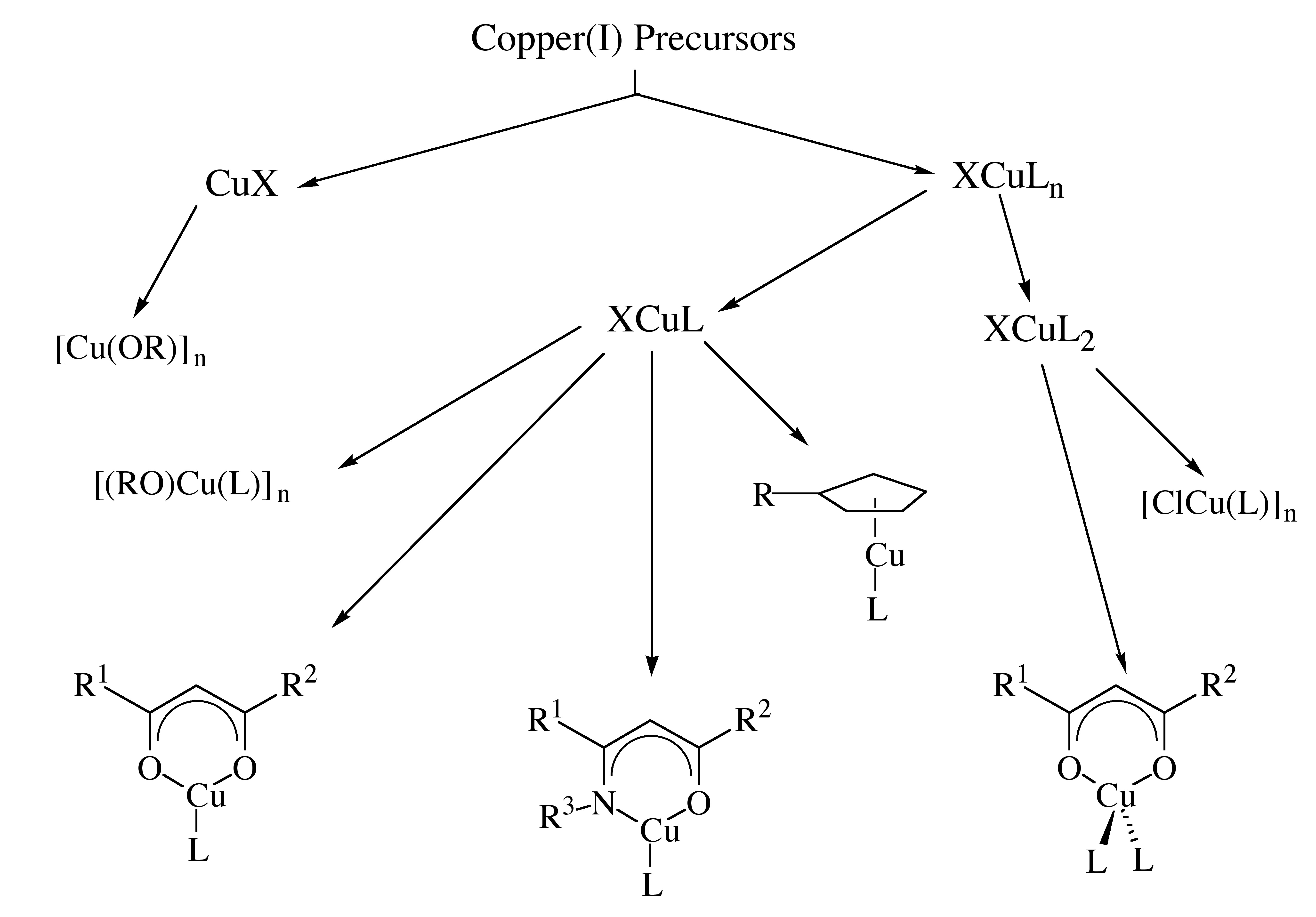
Compounds of general formula CuX are likely to be oligomeric resulting in a relatively low vapor pressure. The presence of a neutral donor ligand, L, is likely to reduce the extent of oligomerization compared to CuX by occupying vacant coordination sites. Metal alkoxide compounds are expected to undergo thermal decomposition by cleavage of either M-O or O-C bonds.
Organo-copper(I) compounds, RCuL, where R is alkyl, are thermally unstable, but cyclopentadienyl compounds are likely to be more robust due to the π-bonding of the cyclopentadienyl ligand to the copper center. At the same time, the cyclopentadienyl ligand is sterically demanding, occupies three coordination sites at the metal center, and thereby reduces the desire for oligomerization. In general, a cyclopentadienyl ligand is a poor choice to support CVD precursors, especially with electropositive metals, because this ligand is unlikely to be liable. Compounds in the family XCuL 2 , where X is a halide and L is a triorganophosphine, exhibit relatively high volatility but are thermally stable with respect to formation of copper at low temperatures. These species are therefore suitable as products of etching reactions of copper films.

Notification Switch
Would you like to follow the 'Chemistry of electronic materials' conversation and receive update notifications?