| << Chapter < Page | Chapter >> Page > |
Prior Knowledge
Investigation of physical qualities of elements
Your educator will help you to investigate the physical features of individual elements and the changes that occur when they combine.
1. Describe the iron filings:
2. Describe the sulphur:
3. Is the iron attracted by the magnet?
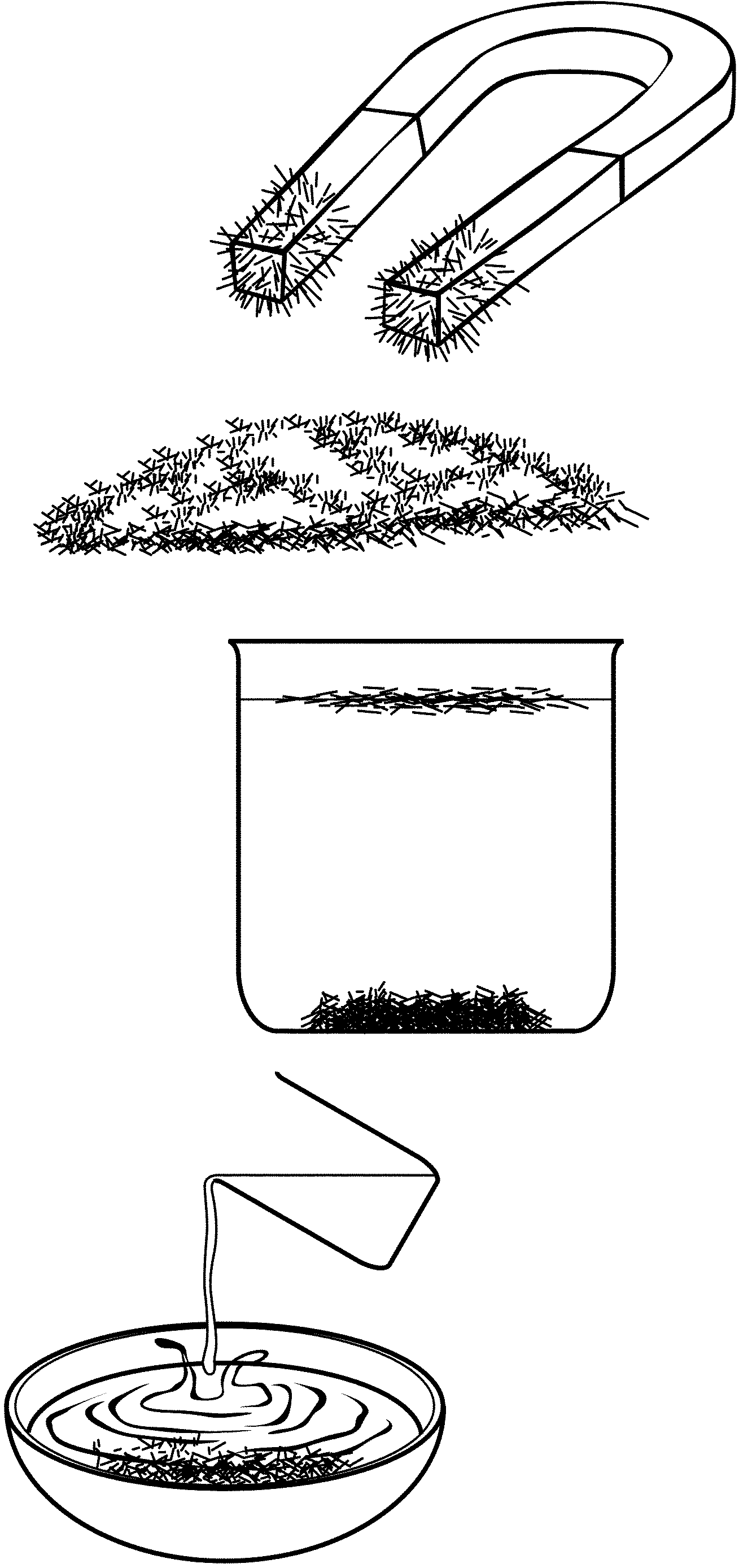
5. What do you deduce from this?
6. Add a little carbon bisulphide (CS 2 ) to the iron.
What happens?
7. Add a little carbon bisulphide (CS 2 ) to the sulphur.
What happens?
8. What do you deduce from this?
Pour the contents of the test tubes into two watch-glasses.
9. What do you see when the carbon bisulphide has evaporated?
Investigation of physical qualities of mixtures
Add iron filings to the sulphur:
1. What does the mixture look like?
2. What happens when the magnet is brought closer to the mixture?
3. What can you deduce from this?
4. Now add CS 2 to the mixture. What happens?
5. Can you separate the mixture?
6. Suggest two methods of separation
1. what happens when iron and sulphur are heated together?
2. Is a new substance formed? Explain your answer?
3. Has the substance retained any magnetic qualities?
4. Is this substance soluble in carbon bisulphide?
5. Can the substance be reconverted to the original elements?
6. What was required for the reaction to occur?
7. What kind of transfer of energy took place?
:
Assessment of experiment
Were you able to plan the steps (AS 1.1), execute them (AS 1.2) and communicate your findings (AS 1.3)?
[LO 1.1; LO 1.2; LO 1.3]
Learning outcome 1: Scientific investigations
The learner will be able to act confidently on curiosity about natural phenomena, and to investigate relationships and solve problems in scientific, technological and environmental contexts.
ASSESSMENT STANDARD: We know this when the learner
1.1 is able to plan investigations;
1.2 is able to execute an investigation and collect data;
INVESTIGATION INTO PHYSICAL QUALITIES OF ELEMENTS
1. grey, hard
2. yellow powder
3. yes
4. no
5. Metals are magnetic.
6. nothing
7. sulphur dissolves
8. Metals do not dissolve in carbon bisulphide.
9. crystals – sulphur
INVESTIGATION INTO PHYSICAL QUALITIES OF MIXTURES
1. yellow-grey
2. Only iron filings are attracted.
3. Only metal is magnetic.
4. Only sulphur dissolves.
5. yes
6. Dissolves S and evaporates again – crystals back on magnet.
CLASS ACTIVITY: DEMONSTRATION
1. Fused into a new substance.
2. yes – looks different; own qualities
3. no
4. no
5. yes, with effort
6. heat
7. heat – chemicals
8. Complete the following comparative table.

Notification Switch
Would you like to follow the 'Natural sciences grade 8' conversation and receive update notifications?