| << Chapter < Page | Chapter >> Page > |
The three lighter boron trihalides, BX 3 (X = F, Cl, Br) form stable adducts with common Lewis bases. Their relative Lewis acidities can be evaluated in terms of the relative exothermicity of the adduct-forming reaction:
This trend is opposite to that expected based upon the electronegativity of the halogens. The best explanation of this trend takes into account the extent of π-donation that occurs between the filled lone pair orbital on the halogens and the empty p-orbital on the planar boron ( [link] ). As such, the greater the π-bonding the more stable the planar BX 3 configuration as opposed to the pyramidalization of the BX 3 moiety upon formation of a Lewis acid-base complex, [link] .
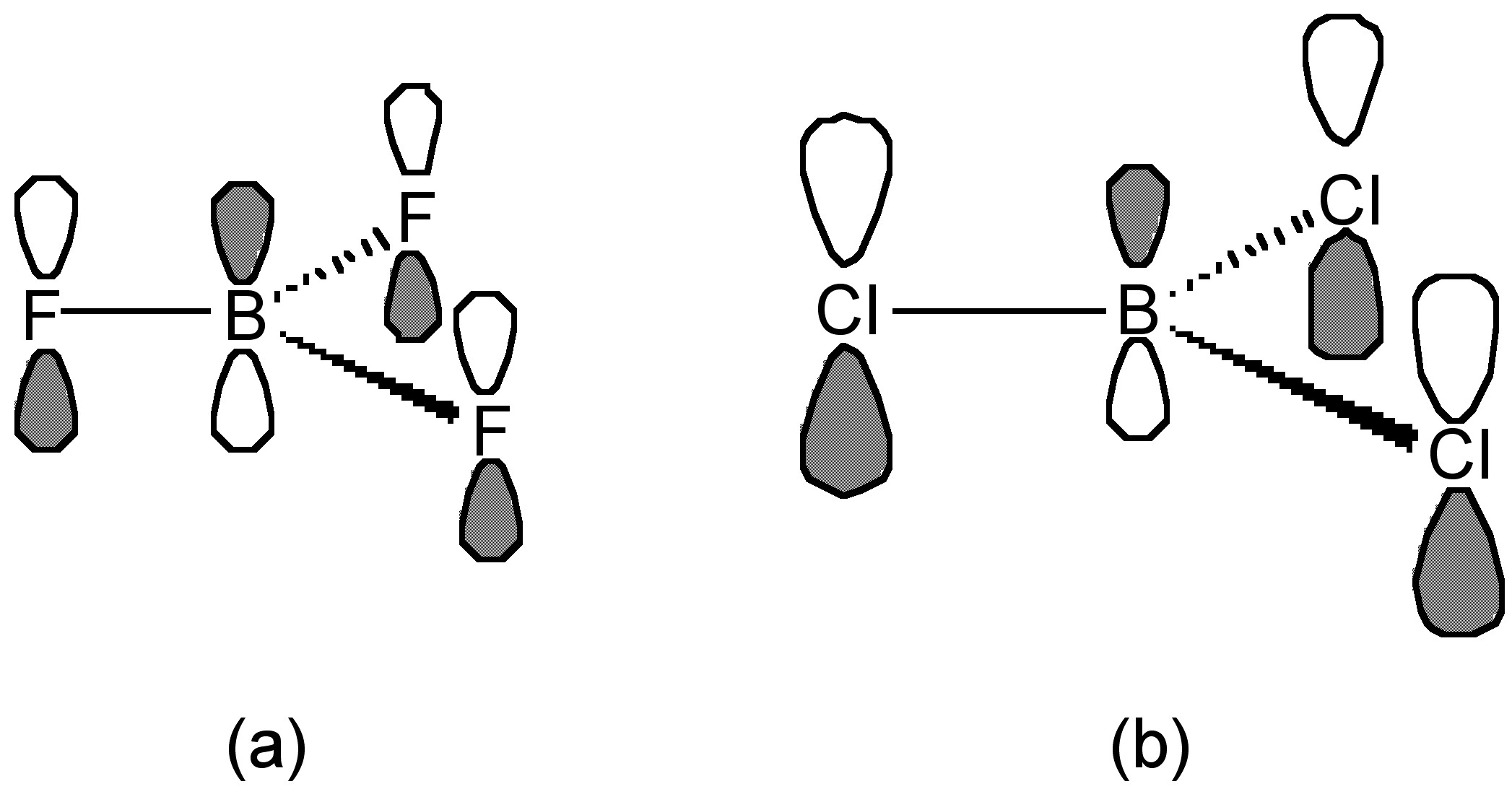
The criteria for evaluating the relative strength of π-bonding are not clear, however, one suggestion is that the F atom is small compared to the Cl atom, and the lone pair electron in p z of F is readily and easily donated and overlapped to empty p z orbital of boron ( [link] a). In contrast, the overlap for the large (diffuse) p-orbitals on the chlorine is poorer ( [link] b). As a result, the π-donation of F is greater than that of Cl. Interestingly, as may be seen from [link] , any difference in B-X bond length does not follow the expected trend associated with shortening of the B-X bond with stronger π-bonding. In fact the B-Br distance is the most shortened as compared to that expected from the covalent radii ( [link] ).
| Compound | B-X (Å) | X covalent radius (Å) | Sum of covalent radii (Å) a | Δ (Å) |
| BF 3 | 1.313 | 0.57(3) | 1.41 | 0.097 |
| BCl 3 | 1.75 | 1.02(4) | 1.86 | 0.11 |
| BBr 3 | 1.898 | 1.20(3) | 2.04 | 0.142 |
| BI 3 | 2.125 | 1.39(3) | 2.23 | 0.105 |
At the simplest level the requirements for bonding to occur based upon the molecular or atomic orbital are:
In the case of the boron trihalides, the direction (parallel) and symmetry (p-orbitals) are the same, and the only significant difference will be the relative energy of the donor orbitals (i.e., the lone pair on the halogen) and the extent of the overlap. The latter will be dependant on the B-X bond length (the shorter the bond the greater the potential overlap) and the diffusion of the orbitals (the less diffuse the orbitals the better the overlap). Both of these factors will benefit B-F over B-Cl and B-Br. Thus, the extent of potential overlap would follow the order: [link] . Despite these considerations, it is still unclear of the exact details of the rationalization of the low Lewis basicity of BF 3 as compared to BCl 3 and BBr 3 .
The trihalides all form Lewis acid-base complexes with halide anions, [link] , and as such salts of BF 4 - , AlCl 4 - , GaCl 4 - , and InCl 4 - are common.
In the case of gallium the Ga 2 Cl 7 - anion ( [link] ) is formed from the equilibrium:

Notification Switch
Would you like to follow the 'Chemistry of the main group elements' conversation and receive update notifications?