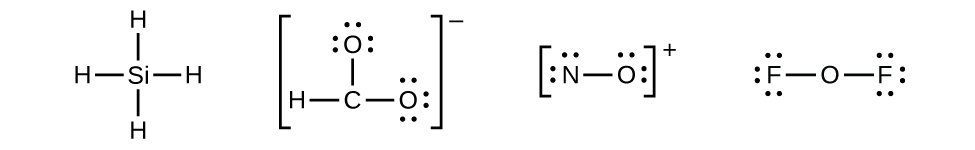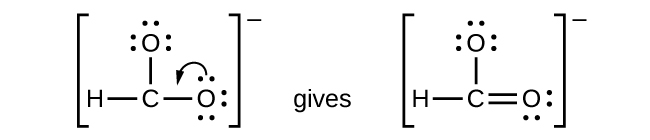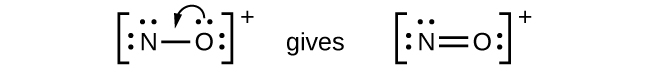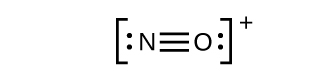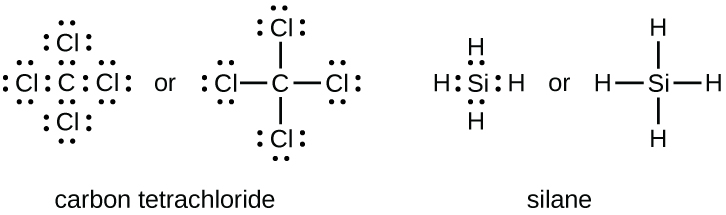
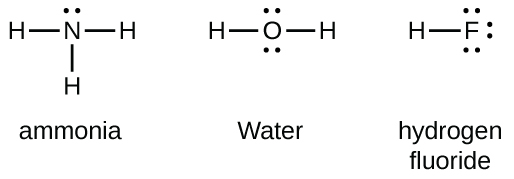
Group 15 elements such as nitrogen have five valence electrons in the atomic Lewis symbol: one lone pair and three unpaired electrons. To obtain an octet, these atoms form three covalent bonds, as in NH
3 (ammonia). Oxygen and other atoms in group 16 obtain an octet by forming two covalent bonds:
Double and triple bonds
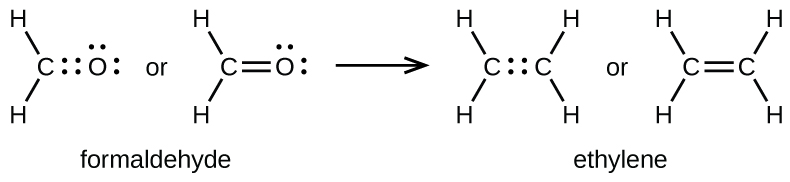
As previously mentioned, when a pair of atoms shares one pair of electrons, we call this a single bond. However, a pair of atoms may need to share more than one pair of electrons in order to achieve the requisite octet. A
double bond forms when two pairs of electrons are shared between a pair of atoms, as between the carbon and oxygen atoms in CH
2 O (formaldehyde) and between the two carbon atoms in C
2 H
4 (ethylene):
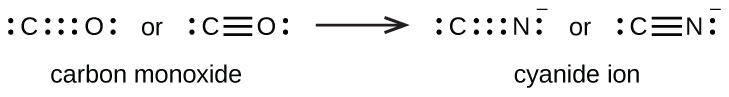
A
triple bond forms when three electron pairs are shared by a pair of atoms, as in carbon monoxide (CO) and the cyanide ion (CN
– ):
Writing lewis structures with the octet rule
For very simple molecules and molecular ions, we can write the Lewis structures by merely pairing up the unpaired electrons on the constituent atoms. See these examples:
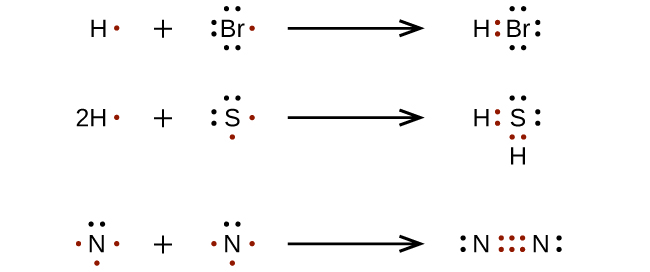
For more complicated molecules and molecular ions, it is helpful to follow the step-by-step procedure outlined here:
- Determine the total number of valence (outer shell) electrons. For cations, subtract one electron for each positive charge. For anions, add one electron for each negative charge.
- Draw a skeleton structure of the molecule or ion, arranging the atoms around a central atom. (Generally, the least electronegative element should be placed in the center.) Connect each atom to the central atom with a single bond (one electron pair).
- Distribute the remaining electrons as lone pairs on the terminal atoms (except hydrogen), completing an octet around each atom.
- Place all remaining electrons on the central atom.
- Rearrange the electrons of the outer atoms to make multiple bonds with the central atom in order to obtain octets wherever possible.
Let us determine the Lewis structures of SiH
4 ,
NO
+ , and OF
2 as examples in following this procedure:
- Determine the total number of valence (outer shell) electrons in the molecule or ion.
- For a molecule, we add the number of valence electrons on each atom in the molecule:
- For a
negative ion , such as
we add the number of valence electrons on the atoms to the number of negative charges on the ion (one electron is gained for each single negative charge):
- For a
positive ion , such as NO
+ , we add the number of valence electrons on the atoms in the ion and then subtract the number of positive charges on the ion (one electron is lost for each single positive charge) from the total number of valence electrons:
- Since OF
2 is a neutral molecule, we simply add the number of valence electrons:
- Draw a skeleton structure of the molecule or ion, arranging the atoms around a central atom and connecting each atom to the central atom with a single (one electron pair) bond. (Note that we denote ions with brackets around the structure, indicating the charge outside the brackets:)
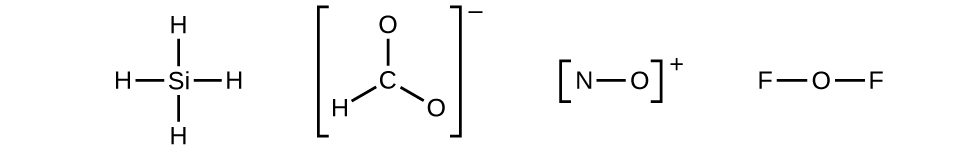
When several arrangements of atoms are possible, as for
we must use experimental evidence to choose the correct one. In general, the less electronegative elements are more likely to be central atoms. In
the less electronegative carbon atom occupies the central position with the oxygen and hydrogen atoms surrounding it. Other examples include P in POCl
3 , S in SO
2 , and Cl in
An exception is that hydrogen is almost never a central atom. As the most electronegative element, fluorine also cannot be a central atom.
- Distribute the remaining electrons as lone pairs on the terminal atoms (except hydrogen) to complete their valence shells with an octet of electrons.
- There are no remaining electrons on SiH
4 , so it is unchanged:
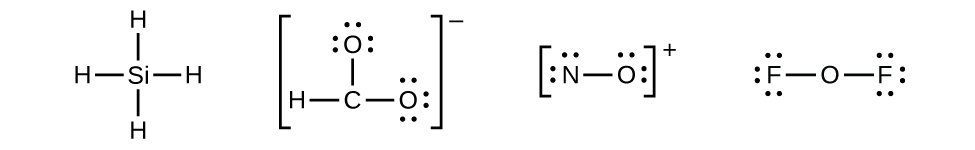
- Place all remaining electrons on the central atom.
- For SiH
4 ,
and NO
+ , there are no remaining electrons; we already placed all of the electrons determined in Step 1.
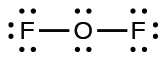
- For OF
2 , we had 16 electrons remaining in Step 3, and we placed 12, leaving 4 to be placed on the central atom:
- Rearrange the electrons of the outer atoms to make multiple bonds with the central atom in order to obtain octets wherever possible.
- SiH
4 : Si already has an octet, so nothing needs to be done.
-
We have distributed the valence electrons as lone pairs on the oxygen atoms, but the carbon atom lacks an octet:
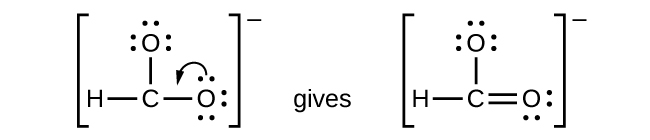
- NO
+ : For this ion, we added eight valence electrons, but neither atom has an octet. We cannot add any more electrons since we have already used the total that we found in Step 1, so we must move electrons to form a multiple bond:
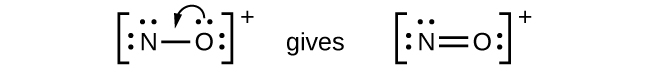
This still does not produce an octet, so we must move another pair, forming a triple bond:
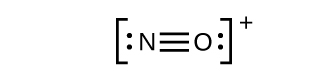
- In OF
2 , each atom has an octet as drawn, so nothing changes.