| << Chapter < Page | Chapter >> Page > |
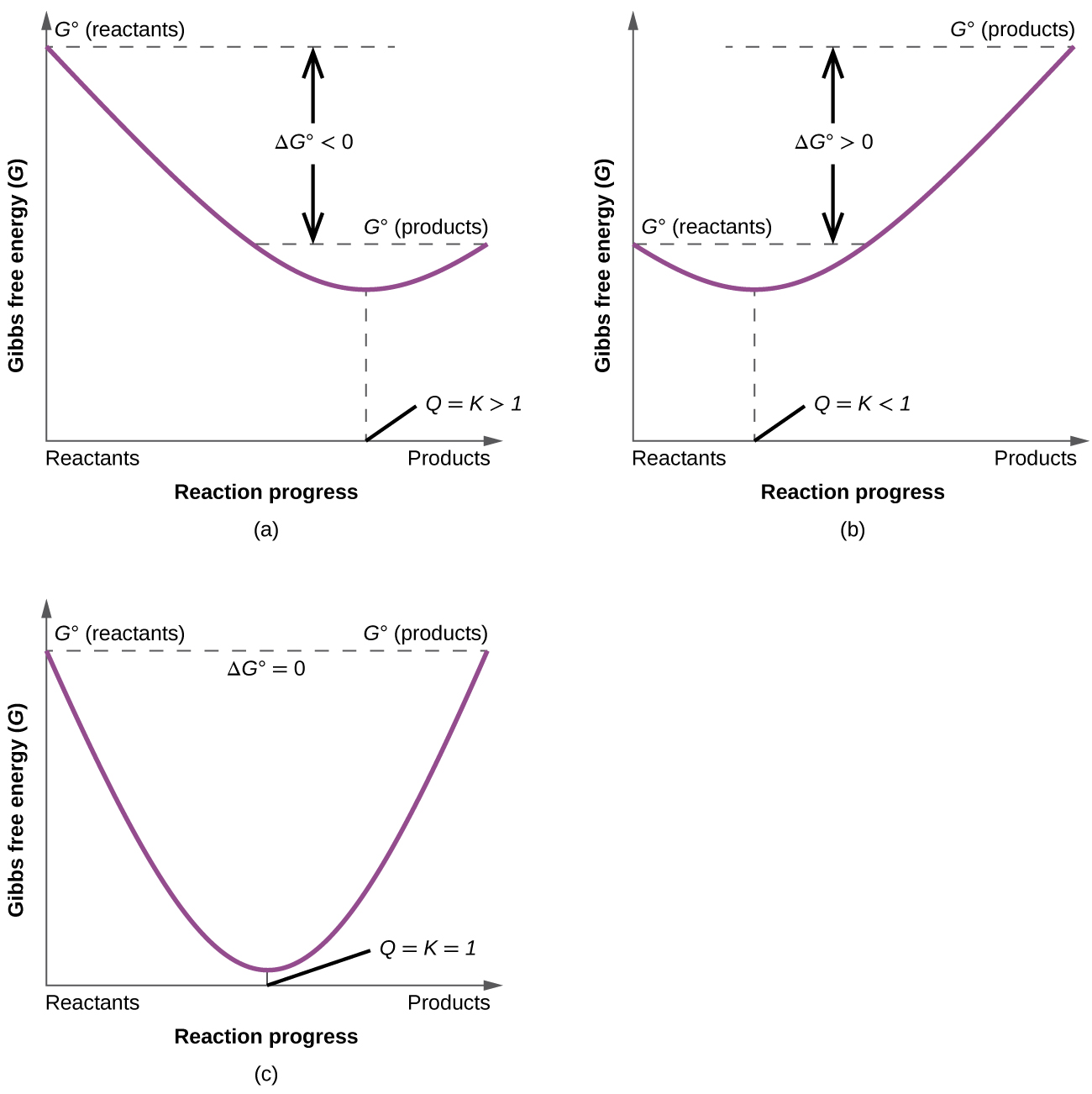
Gibbs free energy ( G ) is a state function defined with regard to system quantities only and may be used to predict the spontaneity of a process. A negative value for Δ G indicates a spontaneous process; a positive Δ G indicates a nonspontaneous process; and a Δ G of zero indicates that the system is at equilibrium. A number of approaches to the computation of free energy changes are possible.
What is the difference between Δ G , Δ G °, and for a chemical change?
A reactions has = 100 kJ/mol and Is the reaction spontaneous at room temperature? If not, under what temperature conditions will it become spontaneous?
The reaction is nonspontaneous at room temperature.
Above 400 K, Δ
G will become negative, and the reaction will become spontaneous.
Explain what happens as a reaction starts with Δ G <0 (negative) and reaches the point where Δ G = 0.
Use the standard free energy of formation data in Appendix G to determine the free energy change for each of the following reactions, which are run under standard state conditions and 25 °C. Identify each as either spontaneous or nonspontaneous at these conditions.
(a)
(b)
(c)
(d)
(e)
(f)
(a) 465.1 kJ nonspontaneous; (b) −106.86 kJ spontaneous; (c) −53.6 kJ spontaneous; (d) −83.4 kJ spontaneous; (e) −406.7 kJ spontaneous; (f) −30.0 kJ spontaneous
Use the standard free energy data in Appendix G to determine the free energy change for each of the following reactions, which are run under standard state conditions and 25 °C. Identify each as either spontaneous or nonspontaneous at these conditions.
(a)
(b)
(c)
(d)
(e)
(f)
Given:
(a) Determine the standard free energy of formation, for phosphoric acid.
(b) How does your calculated result compare to the value in Appendix G ? Explain.
(a) −1124.3 kJ/mol for the standard free energy change. (b) The calculation agrees with the value in Appendix G because free energy is a state function (just like the enthalpy and entropy), so its change depends only on the initial and final states, not the path between them.
Is the formation of ozone (O 3 ( g )) from oxygen (O 2 ( g )) spontaneous at room temperature under standard state conditions?
Consider the decomposition of red mercury(II) oxide under standard state conditions.
(a) Is the decomposition spontaneous under standard state conditions?
(b) Above what temperature does the reaction become spontaneous?
(a) The reaction is nonspontaneous; (b) Above 566 °C the process is spontaneous.

Notification Switch
Would you like to follow the 'Chemistry' conversation and receive update notifications?