| << Chapter < Page | Chapter >> Page > |
Thinking in terms of overlapping atomic orbitals is one way for us to explain how chemical bonds form in diatomic molecules. However, to understand how molecules with more than two atoms form stable bonds, we require a more detailed model. As an example, let us consider the water molecule, in which we have one oxygen atom bonding to two hydrogen atoms. Oxygen has the electron configuration 1 s 2 2 s 2 2 p 4 , with two unpaired electrons (one in each of the two 2 p orbitals). Valence bond theory would predict that the two O–H bonds form from the overlap of these two 2 p orbitals with the 1 s orbitals of the hydrogen atoms. If this were the case, the bond angle would be 90°, as shown in [link] , because p orbitals are perpendicular to each other. Experimental evidence shows that the bond angle is 104.5°, not 90°. The prediction of the valence bond theory model does not match the real-world observations of a water molecule; a different model is needed.
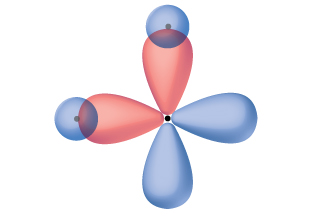
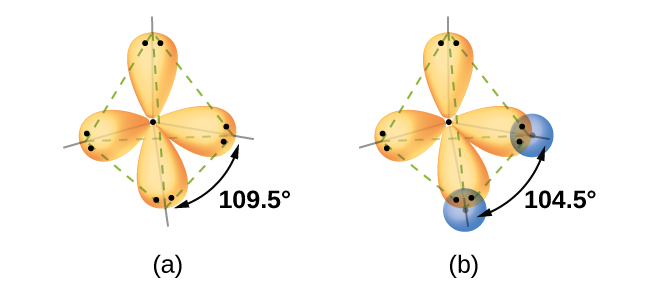
Quantum-mechanical calculations suggest why the observed bond angles in H 2 O differ from those predicted by the overlap of the 1 s orbital of the hydrogen atoms with the 2 p orbitals of the oxygen atom. The mathematical expression known as the wave function, ψ , contains information about each orbital and the wavelike properties of electrons in an isolated atom. When atoms are bound together in a molecule, the wave functions combine to produce new mathematical descriptions that have different shapes. This process of combining the wave functions for atomic orbitals is called hybridization and is mathematically accomplished by the linear combination of atomic orbitals , LCAO, (a technique that we will encounter again later). The new orbitals that result are called hybrid orbitals . The valence orbitals in an isolated oxygen atom are a 2 s orbital and three 2 p orbitals. The valence orbitals in an oxygen atom in a water molecule differ; they consist of four equivalent hybrid orbitals that point approximately toward the corners of a tetrahedron ( [link] ). Consequently, the overlap of the O and H orbitals should result in a tetrahedral bond angle (109.5°). The observed angle of 104.5° is experimental evidence for which quantum-mechanical calculations give a useful explanation: Valence bond theory must include a hybridization component to give accurate predictions.

Notification Switch
Would you like to follow the 'Chemistry' conversation and receive update notifications?