| << Chapter < Page | Chapter >> Page > |
| Average Bond Lengths and Bond Energies for Some Common Bonds | ||
|---|---|---|
| Bond | Bond Length (Å) | Bond Energy (kJ/mol) |
| C–C | 1.54 | 345 |
| 1.34 | 611 | |
| 1.20 | 837 | |
| C–N | 1.43 | 290 |
| 1.38 | 615 | |
| 1.16 | 891 | |
| C–O | 1.43 | 350 |
| 1.23 | 741 | |
| 1.13 | 1080 |
We can use bond energies to calculate approximate enthalpy changes for reactions where enthalpies of formation are not available. Calculations of this type will also tell us whether a reaction is exothermic or endothermic. An exothermic reaction (Δ H negative, heat produced) results when the bonds in the products are stronger than the bonds in the reactants. An endothermic reaction (Δ H positive, heat absorbed) results when the bonds in the products are weaker than those in the reactants.
The enthalpy change, Δ H , for a chemical reaction is approximately equal to the sum of the energy required to break all bonds in the reactants (energy “in”, positive sign) plus the energy released when all bonds are formed in the products (energy “out,” negative sign). This can be expressed mathematically in the following way:
In this expression, the symbol Ʃ means “the sum of” and D represents the bond energy in kilojoules per mole, which is always a positive number. The bond energy is obtained from a table (like [link] ) and will depend on whether the particular bond is a single, double, or triple bond. Thus, in calculating enthalpies in this manner, it is important that we consider the bonding in all reactants and products. Because D values are typically averages for one type of bond in many different molecules, this calculation provides a rough estimate, not an exact value, for the enthalpy of reaction.
Consider the following reaction:
or
To form two moles of HCl, one mole of H–H bonds and one mole of Cl–Cl bonds must be broken. The energy required to break these bonds is the sum of the bond energy of the H–H bond (436 kJ/mol) and the Cl–Cl bond (243 kJ/mol). During the reaction, two moles of H–Cl bonds are formed (bond energy = 432 kJ/mol), releasing 2 432 kJ; or 864 kJ. Because the bonds in the products are stronger than those in the reactants, the reaction releases more energy than it consumes:
This excess energy is released as heat, so the reaction is exothermic. Appendix G gives a value for the standard molar enthalpy of formation of HCl(g), of –92.307 kJ/mol. Twice that value is –184.6 kJ, which agrees well with the answer obtained earlier for the formation of two moles of HCl.
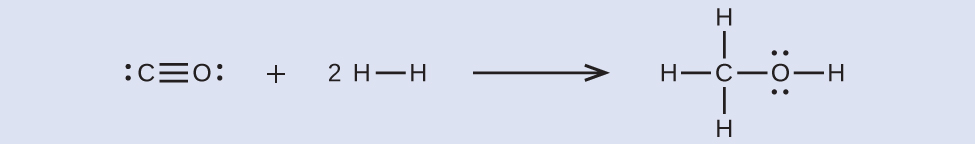
From this, we see that Δ H for this reaction involves the energy required to break a C–O triple bond and two H–H single bonds, as well as the energy produced by the formation of three C–H single bonds, a C–O single bond, and an O–H single bond. We can express this as follows:
Using the bond energy values in [link] , we obtain:
We can compare this value to the value calculated based on data from Appendix G:
Note that there is a fairly significant gap between the values calculated using the two different methods. This occurs because D values are the average of different bond strengths; therefore, they often give only rough agreement with other data.
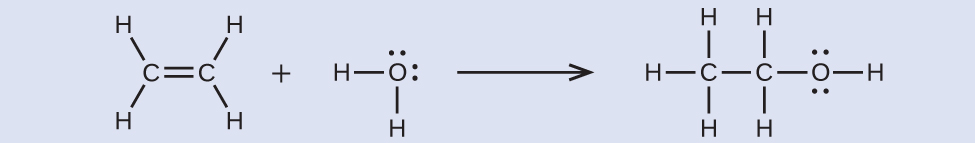
Using the bond energies in [link] , calculate an approximate enthalpy change, Δ H , for this reaction.
–35 kJ

Notification Switch
Would you like to follow the 'Chemistry' conversation and receive update notifications?