| << Chapter < Page | Chapter >> Page > |
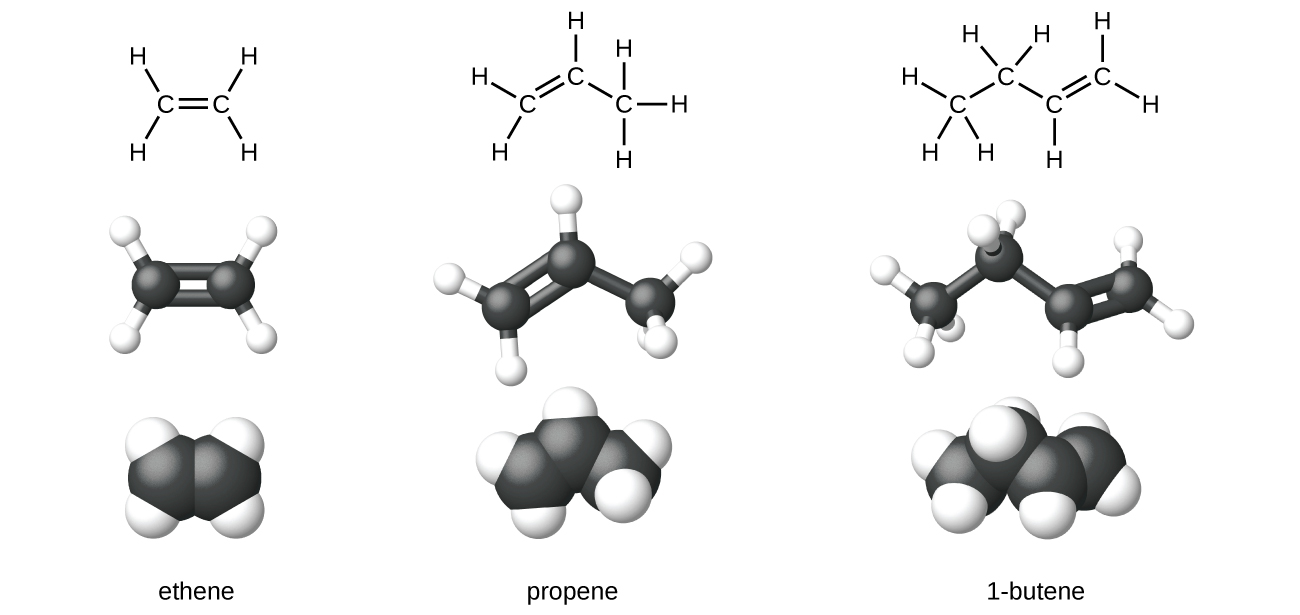
Ethene, C 2 H 4 , is the simplest alkene. Each carbon atom in ethene, commonly called ethylene, has a trigonal planar structure. The second member of the series is propene (propylene) ( [link] ); the butene isomers follow in the series. Four carbon atoms in the chain of butene allows for the formation of isomers based on the position of the double bond, as well as a new form of isomerism.
Ethylene (the common industrial name for ethene) is a basic raw material in the production of polyethylene and other important compounds. Over 135 million tons of ethylene were produced worldwide in 2010 for use in the polymer, petrochemical, and plastic industries. Ethylene is produced industrially in a process called cracking, in which the long hydrocarbon chains in a petroleum mixture are broken into smaller molecules.
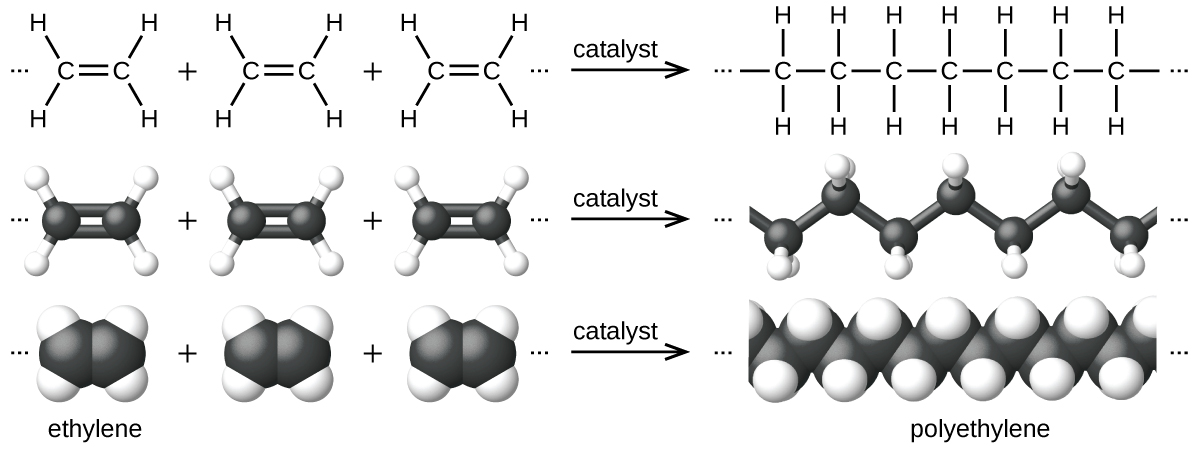
Polymers (from Greek words poly meaning “many” and mer meaning “parts”) are large molecules made up of repeating units, referred to as monomers. Polymers can be natural (starch is a polymer of sugar residues and proteins are polymers of amino acids) or synthetic [like polyethylene, polyvinyl chloride (PVC), and polystyrene]. The variety of structures of polymers translates into a broad range of properties and uses that make them integral parts of our everyday lives. Adding functional groups to the structure of a polymer can result in significantly different properties (see the discussion about Kevlar later in this chapter).
An example of a polymerization reaction is shown in [link] . The monomer ethylene (C 2 H 4 ) is a gas at room temperature, but when polymerized, using a transition metal catalyst, it is transformed into a solid material made up of long chains of –CH 2 – units called polyethylene. Polyethylene is a commodity plastic used primarily for packaging (bags and films).
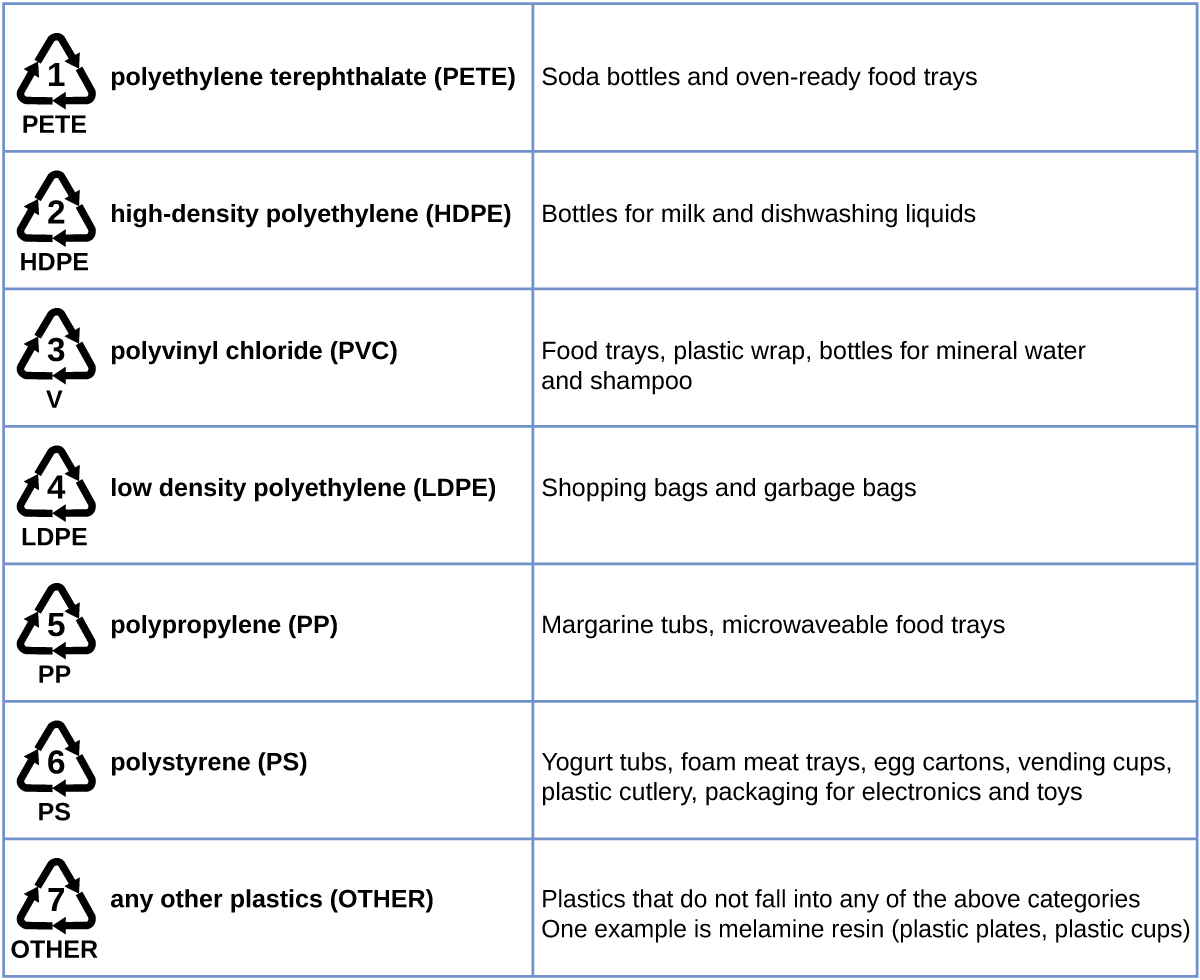
Polyethylene is a member of one subset of synthetic polymers classified as plastics. Plastics are synthetic organic solids that can be molded; they are typically organic polymers with high molecular masses. Most of the monomers that go into common plastics (ethylene, propylene, vinyl chloride, styrene, and ethylene terephthalate) are derived from petrochemicals and are not very biodegradable, making them candidate materials for recycling. Recycling plastics helps minimize the need for using more of the petrochemical supplies and also minimizes the environmental damage caused by throwing away these nonbiodegradable materials.
Plastic recycling is the process of recovering waste, scrap, or used plastics, and reprocessing the material into useful products. For example, polyethylene terephthalate (soft drink bottles) can be melted down and used for plastic furniture, in carpets, or for other applications. Other plastics, like polyethylene (bags) and polypropylene (cups, plastic food containers), can be recycled or reprocessed to be used again. Many areas of the country have recycling programs that focus on one or more of the commodity plastics that have been assigned a recycling code (see [link] ). These operations have been in effect since the 1970s and have made the production of some plastics among the most efficient industrial operations today.

Notification Switch
Would you like to follow the 'Chemistry' conversation and receive update notifications?