| << Chapter < Page | Chapter >> Page > |
A mixture of a weak acid and its conjugate base (or a mixture of a weak base and its conjugate acid) is called a buffer solution, or a buffer . Buffer solutions resist a change in pH when small amounts of a strong acid or a strong base are added ( [link] ). A solution of acetic acid and sodium acetate (CH 3 COOH + CH 3 COONa) is an example of a buffer that consists of a weak acid and its salt. An example of a buffer that consists of a weak base and its salt is a solution of ammonia and ammonium chloride (NH 3 ( aq ) + NH 4 Cl( aq )).

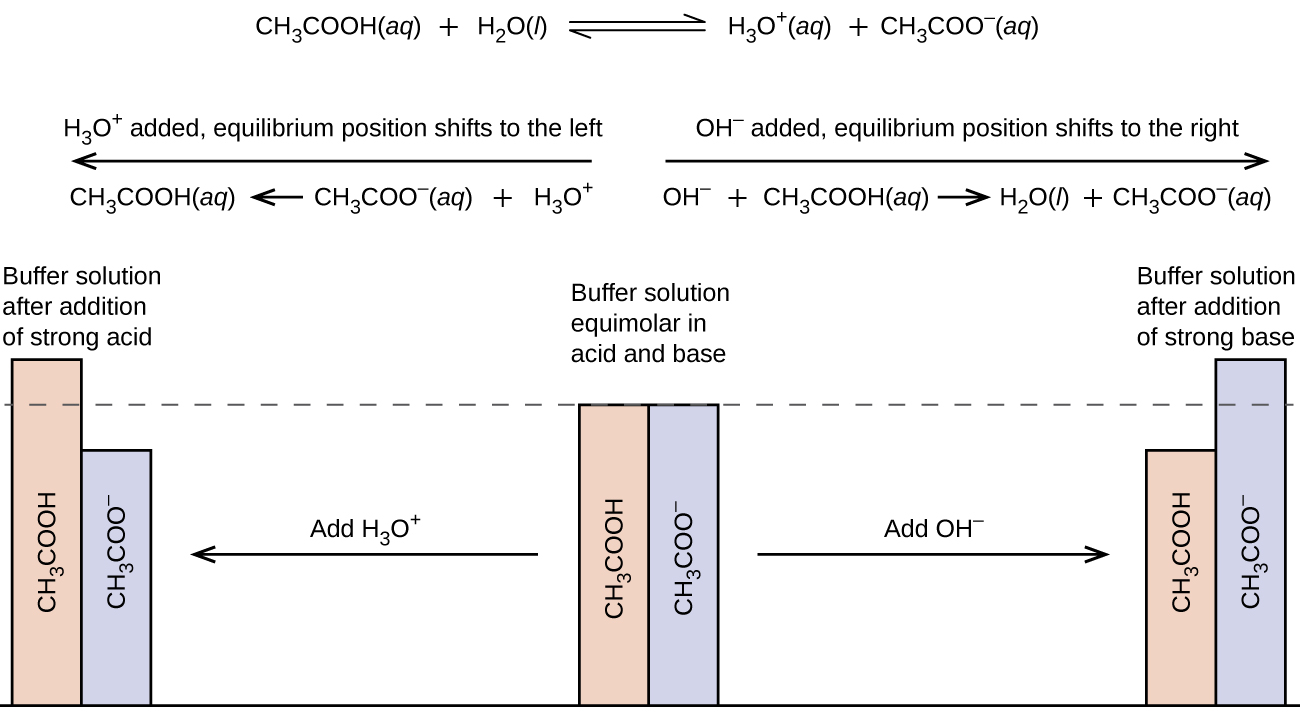
A mixture of acetic acid and sodium acetate is acidic because the K a of acetic acid is greater than the K b of its conjugate base acetate. It is a buffer because it contains both the weak acid and its salt. Hence, it acts to keep the hydronium ion concentration (and the pH) almost constant by the addition of either a small amount of a strong acid or a strong base. If we add a base such as sodium hydroxide, the hydroxide ions react with the few hydronium ions present. Then more of the acetic acid reacts with water, restoring the hydronium ion concentration almost to its original value:
The pH changes very little. If we add an acid such as hydrochloric acid, most of the hydronium ions from the hydrochloric acid combine with acetate ions, forming acetic acid molecules:
Thus, there is very little increase in the concentration of the hydronium ion, and the pH remains practically unchanged ( [link] ).
A mixture of ammonia and ammonium chloride is basic because the K b for ammonia is greater than the K a for the ammonium ion. It is a buffer because it also contains the salt of the weak base. If we add a base (hydroxide ions), ammonium ions in the buffer react with the hydroxide ions to form ammonia and water and reduce the hydroxide ion concentration almost to its original value:
If we add an acid (hydronium ions), ammonia molecules in the buffer mixture react with the hydronium ions to form ammonium ions and reduce the hydronium ion concentration almost to its original value:
The three parts of the following example illustrate the change in pH that accompanies the addition of base to a buffered solution of a weak acid and to an unbuffered solution of a strong acid.

Notification Switch
Would you like to follow the 'Chemistry' conversation and receive update notifications?