| << Chapter < Page | Chapter >> Page > |
| Ionization Constants of Weak Acids | |||
|---|---|---|---|
| Acid | Formula | K a at 25 °C | Lewis Structure |
| acetic | CH 3 CO 2 H | 1.8 10 −5 |
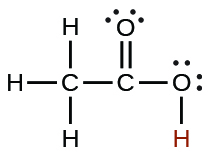 |
| arsenic | H 3 AsO 4 | 5.5 10 −3 |
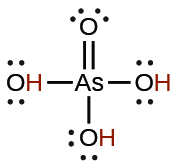 |
 |
1.7 10 −7 | ||
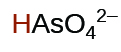 |
5.1 10 −12 | ||
| arsenous | H 3 AsO 3 | 5.1 10 −10 |
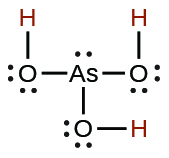 |
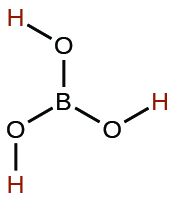
| boric | H 3 BO 3 | 5.4 10 −10 |
 |
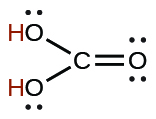
| carbonic | H 2 CO 3 | 4.3 10 −7 |
 |
 |
5.6 10 −11 | ||
| cyanic | H CNO | 2 10 −4 |
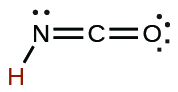 |
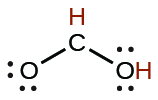
| formic | HCO 2 H | 1.8 10 −4 |
 |
| hydrazoic | H N 3 | 2.5 10 −5 |
 |
| hydrocyanic | H CN | 4.9 10 −10 | |
| hydrofluoric | H F | 3.5 10 −4 | |
| hydrogen peroxide | H 2 O 2 | 2.4 10 −12 |
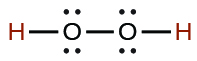 |
| hydrogen selenide | H 2 Se | 1.29 10 −4 | |
| H Se – | 1 10 −12 | ||
| hydrogen sulfate ion |
 |
1.2 10 −2 |
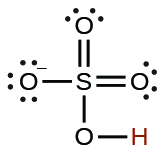 |
| hydrogen sulfide | H 2 S | 8.9 10 −8 | |
| H S – | 1.0 10 −19 | ||
| hydrogen telluride | H 2 Te | 2.3 10 −3 | |
| H Te – | 1.6 10 −11 | ||
| hypobromous | H BrO | 2.8 10 −9 | |
| hypochlorous | H ClO | 2.9 10 −8 | |
| nitrous | H NO 2 | 4.6 10 −4 |
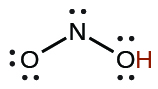 |
| oxalic | H 2 C 2 O 4 | 6.0 10 −2 |
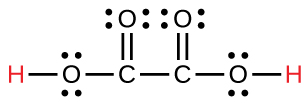 |
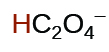 |
6.1 10 −5 | ||
| phosphoric | H 3 PO 4 | 7.5 10 −3 |
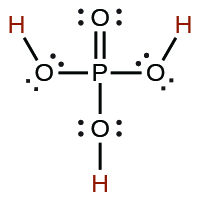 |
 |
6.2 10 −8 | ||
 |
4.2 10 −13 | ||
| phosphorous | H 3 PO 3 | 5 10 −2 |
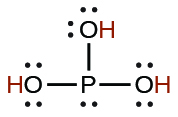 |
 |
2.0 10 −7 | ||
| sulfurous | H 2 SO 3 | 1.6 10 −2 |
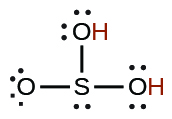 |
 |
6.4 10 −8 |

Notification Switch
Would you like to follow the 'Chemistry' conversation and receive update notifications?