| << Chapter < Page | Chapter >> Page > |
Osmosis is the diffusion of water through a semipermeable membrane according to the concentration gradient of water across the membrane. Whereas diffusion transports material across membranes and within cells, osmosis transports only water across a membrane and the membrane limits the diffusion of solutes in the water. Osmosis is a special case of diffusion. Water, like other substances, moves from an area of higher concentration to one of lower concentration. Imagine a beaker with a semipermeable membrane, separating the two sides or halves ( [link] ). On both sides of the membrane, the water level is the same, but there are different concentrations on each side of a dissolved substance, or solute , that cannot cross the membrane. If the volume of the water is the same, but the concentrations of solute are different, then there are also different concentrations of water, the solvent, on either side of the membrane.
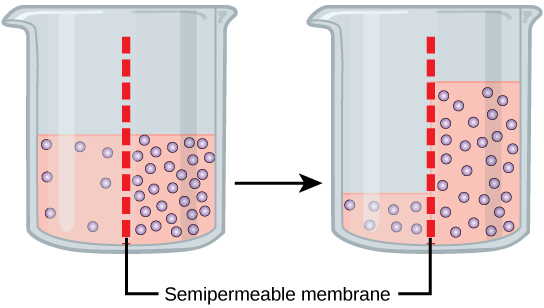
A principle of diffusion is that the molecules move around and will spread evenly throughout the medium if they can. However, only the material capable of getting through the membrane will diffuse through it. In this example, the solute cannot diffuse through the membrane, but the water can. Water has a concentration gradient in this system. Therefore, water will diffuse down its concentration gradient, crossing the membrane to the side where it is less concentrated. This diffusion of water through the membrane—osmosis—will continue until the concentration gradient of water goes to zero. Osmosis proceeds constantly in living systems.
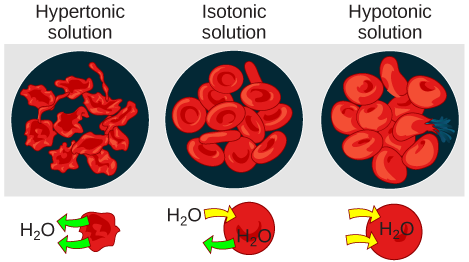
Tonicity describes the amount of solute in a solution. Three terms—hypotonic, isotonic, and hypertonic—are used to relate the concentration of solutes inside of a cell compared to the concentration of solutes in the fluid that contains the cells. In a hypotonic solution, such as tap water, the extracellular fluid has a lower concentration of solutes than the fluid inside the cell, and water enters the cell. (In living systems, the point of reference is always the cytoplasm, so the prefix hypo - means that the extracellular fluid has a lower concentration of solutes, than the cell cytoplasm.) It also means that the extracellular fluid has a higher concentration of water than does the cell. In this situation, water will follow its concentration gradient and enter the cell. This may cause an animal cell to burst, or lyse. Note in these cases that there is always a net movement of water (the solvent) towards the hypertonic solution by the process of osmosis. That is because the hypertonic solution has a lower concentration of water and the solute can't pass through the membrane.
In a hypertonic solution (the prefix hyper - refers to the extracellular fluid having a higher concentration of solutes than the cell’s cytoplasm), the fluid contains less water than the cell does, such as seawater. Because the cell has a lower concentration of solutes, the water will leave the cell. In effect, the solute is drawing the water out of the cell. This may cause an animal cell to shrivel, or crenate.
In an isotonic solution, the extracellular fluid has the same osmolarity as the cell. If the concentration of solutes of the cell matches that of the extracellular fluid, there will be no net movement of water into or out of the cell. Blood cells in hypertonic, isotonic, and hypotonic solutions take on characteristic appearances ( [link] ).
The passive forms of transport, diffusion and osmosis, move material of small molecular weight. Substances diffuse from areas of high concentration to areas of low concentration, and this process continues until the substance is evenly distributed in a system. In solutions of more than one substance, each type of molecule diffuses according to its own concentration gradient. Many factors can affect the rate of diffusion, including concentration gradient, the sizes of the particles that are diffusing, and the temperature of the system.
In living systems, diffusion of substances into and out of cells is mediated by the plasma membrane. Some materials diffuse readily through the membrane, but others are hindered, and their passage is only made possible by protein channels and carriers. The chemistry of living things occurs in aqueous solutions, and balancing the concentrations of those solutions is an ongoing problem. In living systems, diffusion of some substances would be slow or difficult without membrane proteins.
[link] A doctor injects a patient with what he thinks is isotonic saline solution. The patient dies, and autopsy reveals that many red blood cells have been destroyed. Do you think the solution the doctor injected was really isotonic?
[link] No, it must have been hypotonic, as a hypotonic solution would cause water to enter the cells, thereby making them burst.

Notification Switch
Would you like to follow the 'Human biology' conversation and receive update notifications?