| << Chapter < Page | Chapter >> Page > |
The purpose of the respiratory system is to perform gas exchange. Inhaling provides air to the alveoli for this gas exchange process. At the respiratory membrane, where the alveolar and capillary walls meet, gases move across the membranes, with oxygen entering the bloodstream and carbon dioxide exiting. It is through this mechanism that blood is oxygenated and carbon dioxide, the waste product of cellular respiration, is removed from the body via exhaling.
In order to understand the mechanisms of gas exchange in the lung, it is important to understand the underlying principles of gases and their behavior. In addition to Boyle’s law, several other gas laws help to describe the behavior of gases.
Gas molecules exert force on the surfaces with which they are in contact; this force is called pressure. In natural systems, gases are normally present as a mixture of different types of molecules. For example, the atmosphere consists of oxygen, nitrogen, carbon dioxide, and other gaseous molecules, and this gaseous mixture exerts a certain pressure referred to as atmospheric pressure ( [link] ). Partial pressure ( P x ) is the pressure of a single type of gas in a mixture of gases. For example, in the atmosphere, oxygen exerts a partial pressure, and nitrogen exerts another partial pressure, independent of the partial pressure of oxygen ( [link] ). Total pressure is the sum of all the partial pressures of a gaseous mixture.
| Partial Pressures of Atmospheric Gases | ||
|---|---|---|
| Gas | Percent of total composition | Partial pressure
(mm Hg) |
| Nitrogen (N 2 ) | 78.6 | 597.4 |
| Oxygen (O 2 ) | 20.9 | 158.8 |
| Water (H 2 O) | 0.04 | 3.0 |
| Carbon dioxide (CO 2 ) | 0.004 | 0.3 |
| Others | 0.0006 | 0.5 |
| Total composition/total atmospheric pressure | 100% | 760.0 |
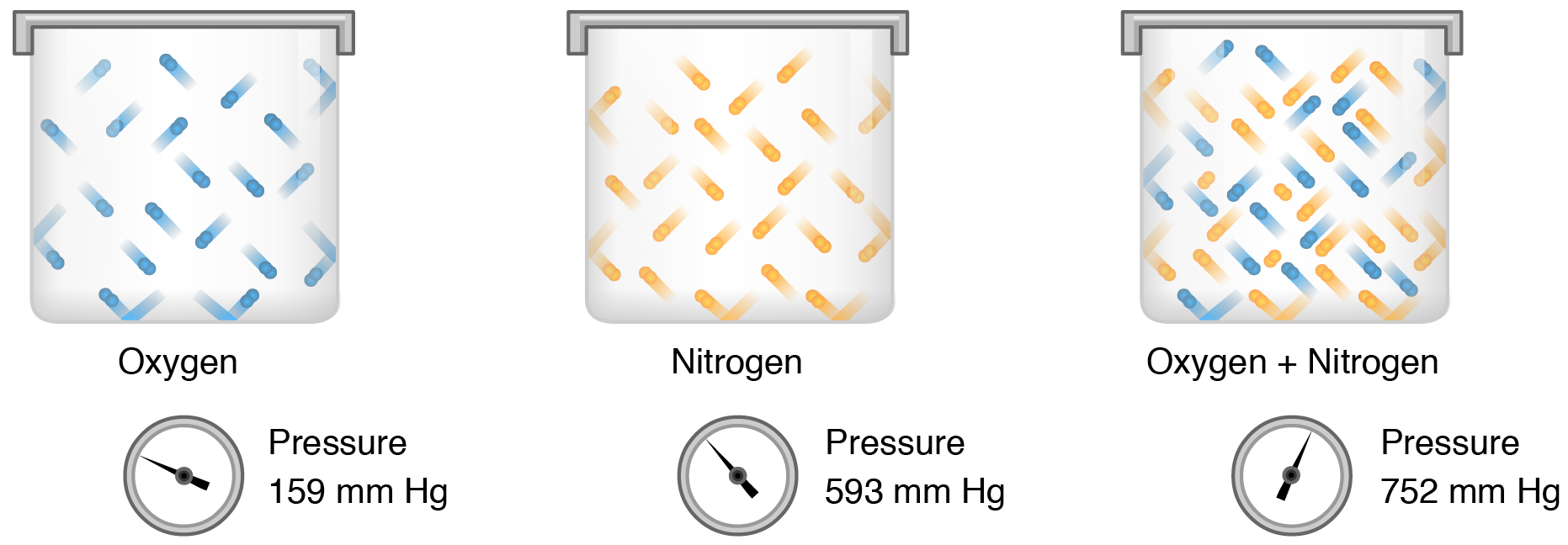
Partial pressure is extremely important in predicting the movement of gases. Recall that gases tend to equalize their pressure in two regions that are connected. A gas will move from an area where its partial pressure is higher to an area where its partial pressure is lower. In addition, the greater the partial pressure difference between the two areas, the more rapid is the movement of gases.
Henry’s law describes the behavior of gases when they come into contact with a liquid, such as blood. Henry’s law states that the concentration of gas in a liquid is directly proportional to the solubility and partial pressure of that gas. The greater the partial pressure of the gas, the greater the number of gas molecules that will dissolve in the liquid. The concentration of the gas in a liquid is also dependent on the solubility of the gas in the liquid. For example, although nitrogen is present in the atmosphere, very little nitrogen dissolves into the blood, because the solubility of nitrogen in blood is very low. The exception to this occurs in scuba divers; the composition of the compressed air that divers breathe causes nitrogen to have a higher partial pressure than normal, causing it to dissolve in the blood in greater amounts than normal. Too much nitrogen in the bloodstream results in a serious condition that can be fatal if not corrected. Gas molecules establish an equilibrium between those molecules dissolved in liquid and those in air.

Notification Switch
Would you like to follow the 'Human biology' conversation and receive update notifications?