| << Chapter < Page | Chapter >> Page > |
A burn results when the skin is damaged by intense heat, radiation, electricity, or chemicals. The damage results in the death of skin cells, which can lead to a massive loss of fluid. Dehydration, electrolyte imbalance, and renal and circulatory failure follow, which can be fatal. Burn patients are treated with intravenous fluids to offset dehydration, as well as intravenous nutrients that enable the body to repair tissues and replace lost proteins. Another serious threat to the lives of burn patients is infection. Burned skin is extremely susceptible to bacteria and other pathogens, due to the loss of protection by intact layers of skin.
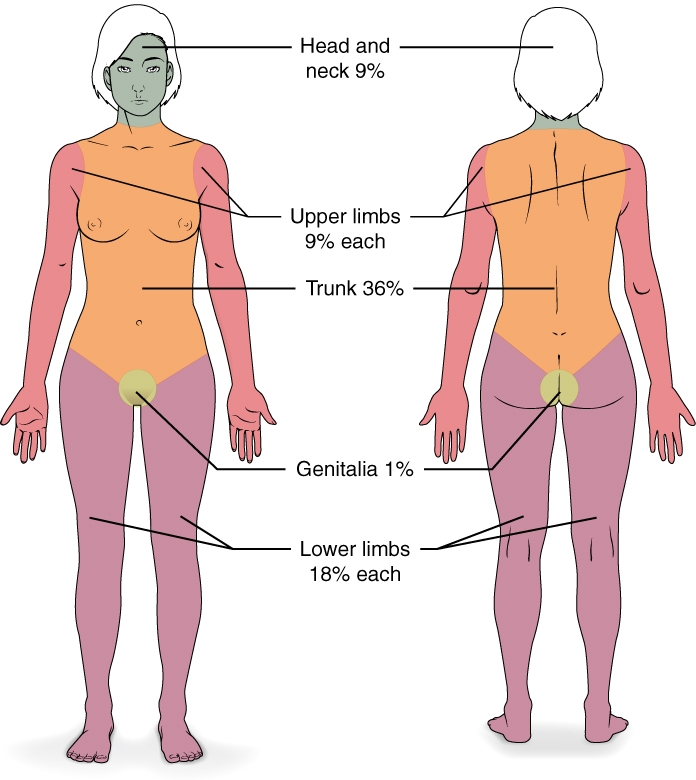
Burns are sometimes measured in terms of the size of the total surface area affected. This is referred to as the “rule of nines,” which associates specific anatomical areas with a percentage that is a factor of nine ( [link] ). Burns are also classified by the degree of their severity. A first-degree burn is a superficial burn that affects only the epidermis. Although the skin may be painful and swollen, these burns typically heal on their own within a few days. Mild sunburn fits into the category of a first-degree burn. A second-degree burn goes deeper and affects both the epidermis and a portion of the dermis. These burns result in swelling and a painful blistering of the skin. It is important to keep the burn site clean and sterile to prevent infection. If this is done, the burn will heal within several weeks. A third-degree burn fully extends into the epidermis and dermis, destroying the tissue and affecting the nerve endings and sensory function. These are serious burns that may appear white, red, or black; they require medical attention and will heal slowly without it. A fourth-degree burn is even more severe, affecting the underlying muscle and bone. Oddly, third and fourth-degree burns are usually not as painful because the nerve endings themselves are damaged. Full-thickness burns cannot be repaired by the body, because the local tissues used for repair are damaged and require excision (debridement), or amputation in severe cases, followed by grafting of the skin from an unaffected part of the body, or from skin grown in tissue culture for grafting purposes.
Skin grafts are required when the damage from trauma or infection cannot be closed with sutures or staples. Watch this video to learn more about skin grafting procedures.
Most cuts or wounds, with the exception of ones that only scratch the surface (the epidermis), lead to scar formation. A scar is collagen-rich skin formed after the process of wound healing that differs from normal skin. Scarring occurs in cases in which there is repair of skin damage, but the skin fails to regenerate the original skin structure. Fibroblasts generate scar tissue in the form of collagen, and the bulk of repair is due to the basket-weave pattern generated by collagen fibers and does not result in regeneration of the typical cellular structure of skin. Instead, the tissue is fibrous in nature and does not allow for the regeneration of accessory structures, such as hair follicles, sweat glands, or sebaceous glands.

Notification Switch
Would you like to follow the 'Anatomy & Physiology' conversation and receive update notifications?