| << Chapter < Page | Chapter >> Page > |
Enzymatic reactions—chemical reactions catalyzed by enzymes—begin when substrates bind to the enzyme. A substrate is a reactant in an enzymatic reaction. This occurs on regions of the enzyme known as active sites ( [link] ). Any given enzyme catalyzes just one type of chemical reaction. This characteristic, called specificity, is due to the fact that a substrate with a particular shape and electrical charge can bind only to an active site corresponding to that substrate.
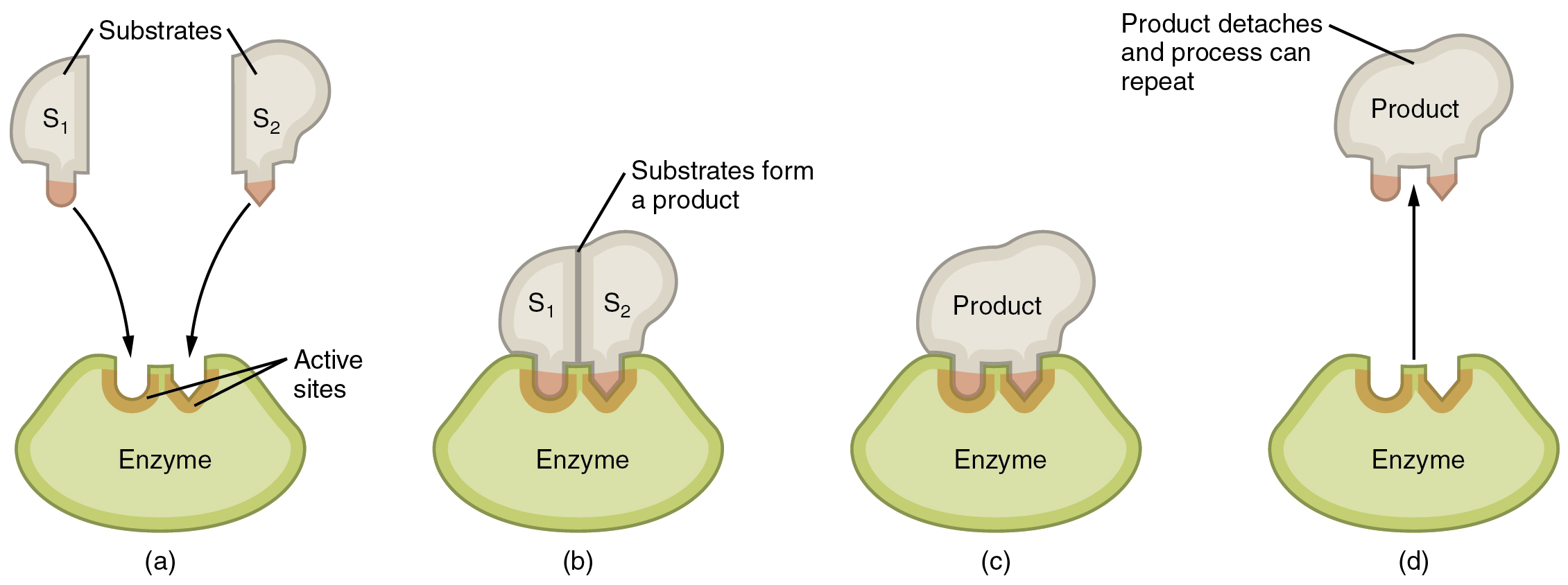
Binding of a substrate produces an enzyme–substrate complex. It is likely that enzymes speed up chemical reactions in part because the enzyme–substrate complex undergoes a set of temporary and reversible changes that cause the substrates to be oriented toward each other in an optimal position to facilitate their interaction. This promotes increased reaction speed. The enzyme then releases the product(s), and resumes its original shape. The enzyme is then free to engage in the process again, and will do so as long as substrate remains.
Advertisements for protein bars, powders, and shakes all say that protein is important in building, repairing, and maintaining muscle tissue, but the truth is that proteins contribute to all body tissues, from the skin to the brain cells. Also, certain proteins act as hormones, chemical messengers that help regulate body functions, For example, growth hormone is important for skeletal growth, among other roles.
As was noted earlier, the basic and acidic components enable proteins to function as buffers in maintaining acid–base balance, but they also help regulate fluid–electrolyte balance. Proteins attract fluid, and a healthy concentration of proteins in the blood, the cells, and the spaces between cells helps ensure a balance of fluids in these various “compartments.” Moreover, proteins in the cell membrane help to transport electrolytes in and out of the cell, keeping these ions in a healthy balance. Like lipids, proteins can bind with carbohydrates. They can thereby produce glycoproteins or proteoglycans, both of which have many functions in the body.
The body can use proteins for energy when carbohydrate and fat intake is inadequate, and stores of glycogen and adipose tissue become depleted. However, since there is no storage site for protein except functional tissues, using protein for energy causes tissue breakdown, and results in body wasting.
The fourth type of organic compound important to human structure and function are the nucleotides ( [link] ). A nucleotide is one of a class of organic compounds composed of three subunits:

Notification Switch
Would you like to follow the 'Anatomy & Physiology' conversation and receive update notifications?