| << Chapter < Page | Chapter >> Page > |
The other major activity in the lungs is the process of respiration, the process of gas exchange. The function of respiration is to provide oxygen for use by body cells during cellular respiration and to eliminate carbon dioxide, a waste product of cellular respiration, from the body. In order for the exchange of oxygen and carbon dioxide to occur, both gases must be transported between the external and internal respiration sites. Although carbon dioxide is more soluble than oxygen in blood, both gases require a specialized transport system for the majority of the gas molecules to be moved between the lungs and other tissues.
Even though oxygen is transported via the blood, you may recall that oxygen is not very soluble in liquids. A small amount of oxygen does dissolve in the blood and is transported in the bloodstream, but it is only about 1.5% of the total amount. The majority of oxygen molecules are carried from the lungs to the body’s tissues by a specialized transport system, which relies on the erythrocyte—the red blood cell. Erythrocytes contain a metalloprotein, hemoglobin, which serves to bind oxygen molecules to the erythrocyte ( [link] ). Heme is the portion of hemoglobin that contains iron, and it is heme that binds oxygen. One hemoglobin molecule contains iron-containing Heme molecules, and because of this, each hemoglobin molecule is capable of carrying up to four molecules of oxygen. As oxygen diffuses across the respiratory membrane from the alveolus to the capillary, it also diffuses into the red blood cell and is bound by hemoglobin. The following reversible chemical reaction describes the production of the final product, oxyhemoglobin (Hb–O 2 ), which is formed when oxygen binds to hemoglobin. Oxyhemoglobin is a bright red-colored molecule that contributes to the bright red color of oxygenated blood.
In this formula, Hb represents reduced hemoglobin, that is, hemoglobin that does not have oxygen bound to it. There are multiple factors involved in how readily heme binds to and dissociates from oxygen, which will be discussed in the subsequent sections.
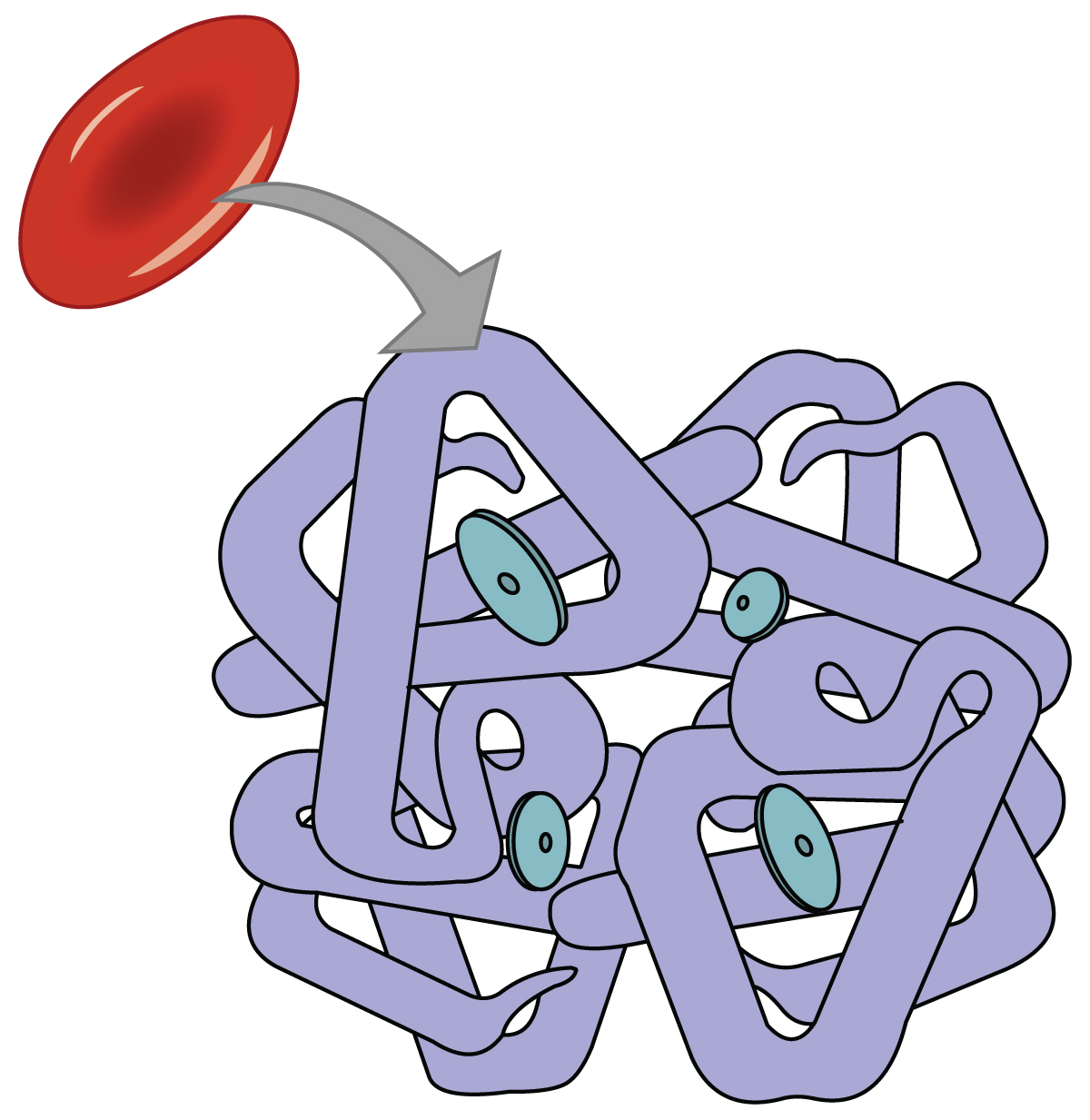
Hemoglobin is composed of subunits, a protein structure that is referred to as a quaternary structure. Each of the four subunits that make up hemoglobin is arranged in a ring-like fashion, with an iron atom covalently bound to the heme in the center of each subunit. Binding of the first oxygen molecule causes a conformational change in hemoglobin that allows the second molecule of oxygen to bind more readily. As each molecule of oxygen is bound, it further facilitates the binding of the next molecule, until all four heme sites are occupied by oxygen. The opposite occurs as well: After the first oxygen molecule dissociates and is “dropped off” at the tissues, the next oxygen molecule dissociates more readily. When all four heme sites are occupied, the hemoglobin is said to be saturated. When one to three heme sites are occupied, the hemoglobin is said to be partially saturated. Therefore, when considering the blood as a whole, the percent of the available heme units that are bound to oxygen at a given time is called hemoglobin saturation. Hemoglobin saturation of 100 percent means that every heme unit in all of the erythrocytes of the body is bound to oxygen. In a healthy individual with normal hemoglobin levels, hemoglobin saturation generally ranges from 95 percent to 99 percent.

Notification Switch
Would you like to follow the 'Anatomy & Physiology' conversation and receive update notifications?