| << Chapter < Page | Chapter >> Page > |
Pulmonary ventilation is the act of breathing, which can be described as the movement of air into and out of the lungs. The major mechanisms that drive pulmonary ventilation are atmospheric pressure ( P atm ); the air pressure within the alveoli, called alveolar pressure ( P alv ); and the pressure within the pleural cavity, called intrapleural pressure ( P ip ).
The alveolar and intrapleural pressures are dependent on certain physical features of the lung. However, the ability to breathe—to have air enter the lungs during inspiration and air leave the lungs during expiration—is dependent on the air pressure of the atmosphere and the air pressure within the lungs.
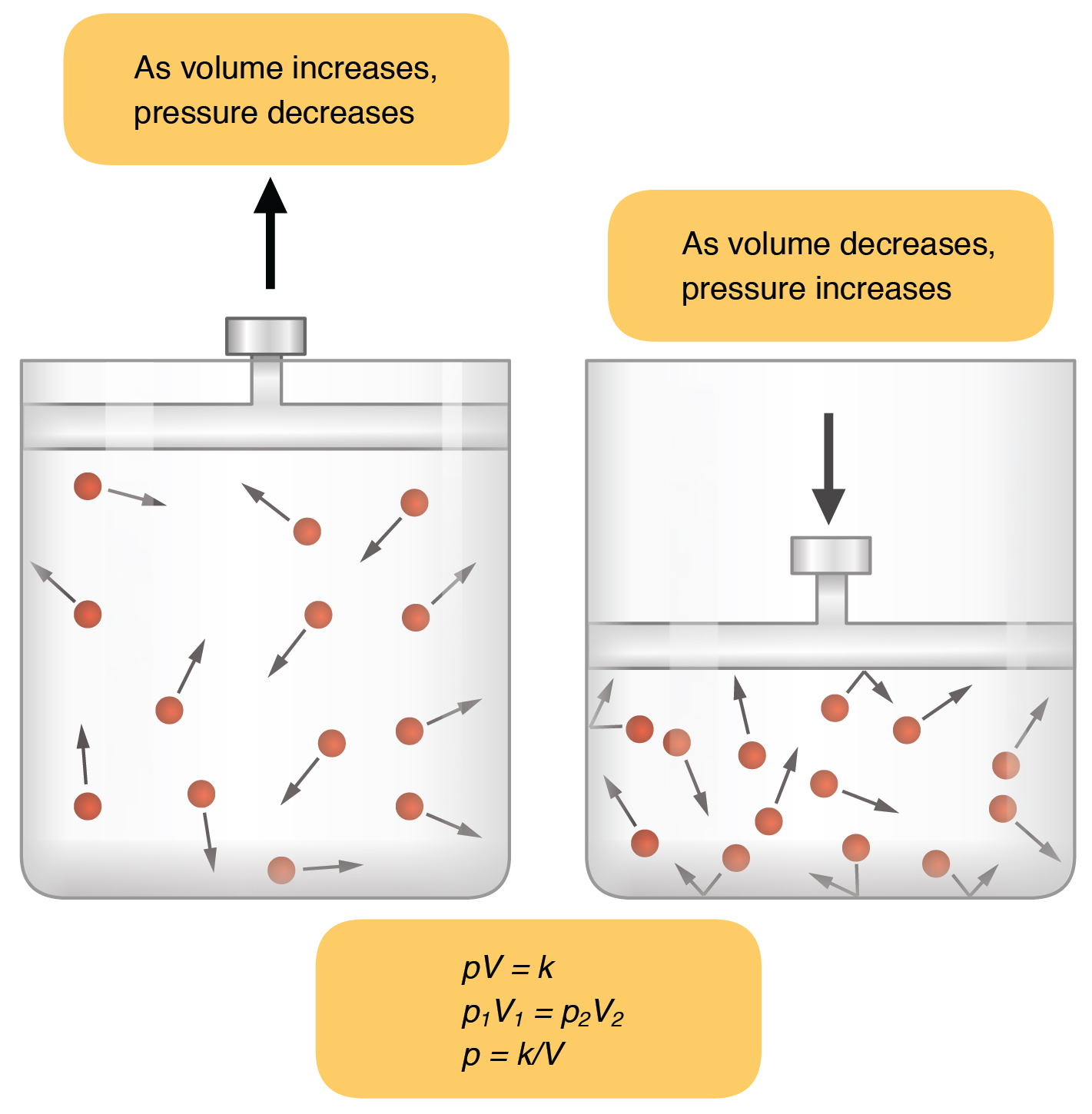
Inspiration (or inhalation) and expiration (or exhalation) are dependent on the differences in pressure between the atmosphere and the lungs. In a gas, pressure is a force created by the movement of gas molecules that are confined. For example, a certain number of gas molecules in a two-liter container has more room than the same number of gas molecules in a one-liter container ( [link] ). In this case, the force exerted by the movement of the gas molecules against the walls of the two-liter container is lower than the force exerted by the gas molecules in the one-liter container. Therefore, the pressure is lower in the two-liter container and higher in the one-liter container. At a constant temperature, changing the volume occupied by the gas changes the pressure, as does changing the number of gas molecules. Boyle’s law describes the relationship between volume and pressure in a gas at a constant temperature. Boyle discovered that the pressure of a gas is inversely proportional to its volume: If volume increases, pressure decreases. Likewise, if volume decreases, pressure increases. Pressure and volume are inversely related ( P = k/ V ). Therefore, the pressure in the one-liter container (one-half the volume of the two-liter container) would be twice the pressure in the two-liter container. Boyle’s law is expressed by the following formula:
In this formula, P 1 represents the initial pressure and V 1 represents the initial volume, whereas the final pressure and volume are represented by P 2 and V 2, respectively. If the two- and one-liter containers were connected by a tube and the volume of one of the containers were changed, then the gases would move from higher pressure (lower volume) to lower pressure (higher volume).

Notification Switch
Would you like to follow the 'Anatomy & Physiology' conversation and receive update notifications?